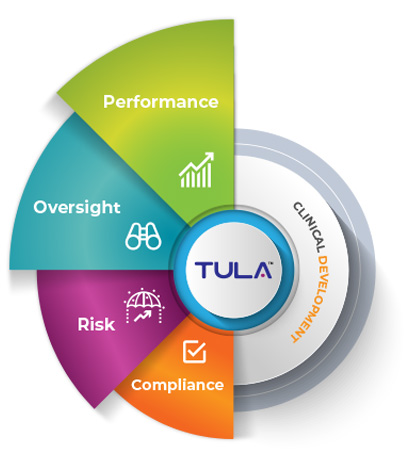
Why Combining Performance, Oversight, Risk and Compliance are very important for your Clinical Development?
Unfortunately, most sponsors and CROs use multiple applications to manage their clinical studies. Integrating multiple applications may be challenging for example reporting through multiple apps, managing data & content across systems, limited collaboration, ease of use, Compliance with standards and the list goes on. To overcome these challenges, you need a single source of truth for your entire Clinical process, underpinning every decision made. TULA helps clinical teams to manage complex processes, identify risks, capture data from applications across the business, automate workflows, and create instant KPI dashboards.
From a quality perspective, TULA provides visibility into performance across study/project portfolio will enable companies to have a single source of truth to utilize for systematic monitoring of portfolio, project and studies performance, risk and compliance policies across. With growing reliance on CROs for development projects having a standardized tool that ensures metrics measurement confidence is paramount to the overall success.
Some example features you may want from TULA solution:
- Get real-time insights into performance across your business. You can track sites / Projects / Programs and share what is working well.
- Systematically keep on track of document and policy life cycles – get the software to do this for you! No more searching through thousands of duplicated documents.
- Drive actions (with timely Alerts & Predictions)
- Bring all your data together, instantly. No more chasing departments for data.
- Give employees the opportunity to speak up about risks they see and identify issues before they occur.
- No siloed systems (RBM, ICH E6 R2 compliance, Central Monitoring & others are all built-in)
- Improves overall efficiency and productivity of organization
TULA makes teams truly Proactive (most teams in most companies are either Reactive or keep them in auto pilot). TULA applies this to all projects, programs, vendors, CROs, and sites all at a time in one platform and liberates this crucial info form trapped siloed teams/functions/systems. No tool in market has TULA’s pre-built ready-to-go functionality. This helps companies use this complex functionality with less than 3 months implementation, all delivered in cloud
Get the latest updates from DDi
Explore Topics
- Automation & AI (6)
- Clinical Automation (9)
- Consumer Health (1)
- IRT & Clinical Supplies (21)
- Labeling (16)
- Regulations (23)
- Regulatory Automation (14)
- Regulatory Biopharma (3)
- Regulatory Content Management (5)
- Regulatory Information Management (21)
- UDI (11)
- Writing (12)
Recent Blogs
 Pharma Workflows with Business…Regulatory Biopharma
Pharma Workflows with Business…Regulatory Biopharma Role of AI in Advancing Pharma…Automation & AI, Clinical Automation
Role of AI in Advancing Pharma…Automation & AI, Clinical Automation Digital RIM for Next-Gen Medic…Regulatory Information Management
Digital RIM for Next-Gen Medic…Regulatory Information Management
Previous Post
Next Post
Related Posts
CONNECT WITH US

Let's talk about how DDi can help you