Publishing Automation –
Reduce Manual efforts, Reworks,
Errors, Cycle time and Cost,
for Pharma & Biotech
Document Publishing is important, and companies optimize this by best practices, outsourcing and some tools. Doesn’t matter what tools or vendors you use currently, probably most of the steps or activities are still largely manual. This may be leading to reworks, longer cycle times, people burn out, additional costs and frustrations.
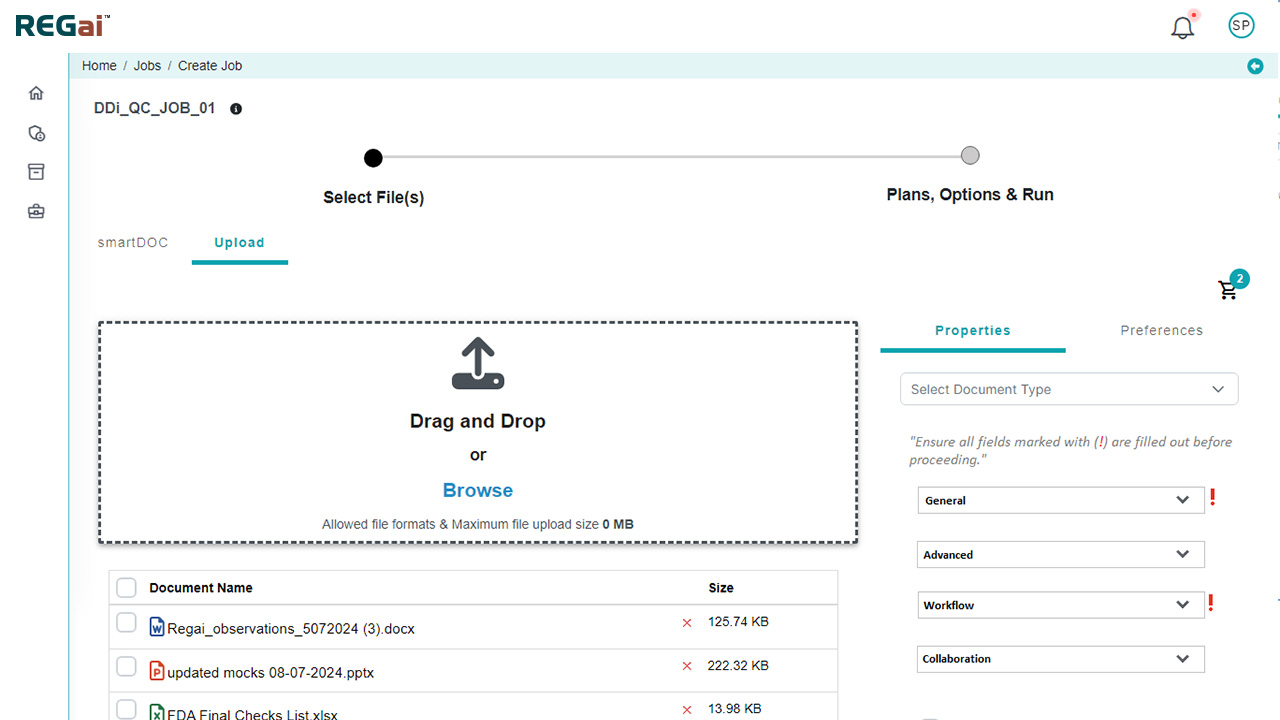
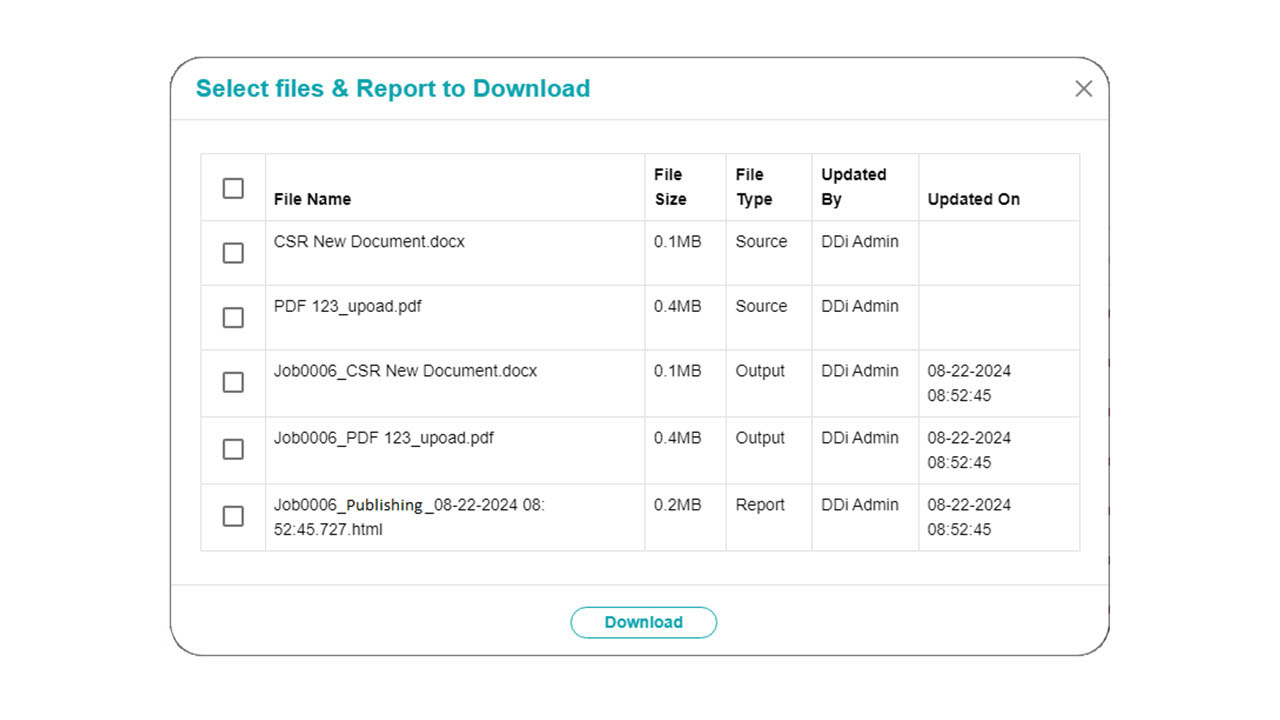
Whether your source files are in MS WORD or PDF, get submission ready files in minutes. With a deep 400 rules library, you pick and create your custom plans with flexibility of applying what/when to your documents. Our solution automates document publishing without disturbing your current eCTD infrastructure and aligns to your current systems and processes by providing different options (local, cloud, plugins, integration)
Time & Cost Savings: 70% and above compared to your current Document publishing costs. Guaranteed!
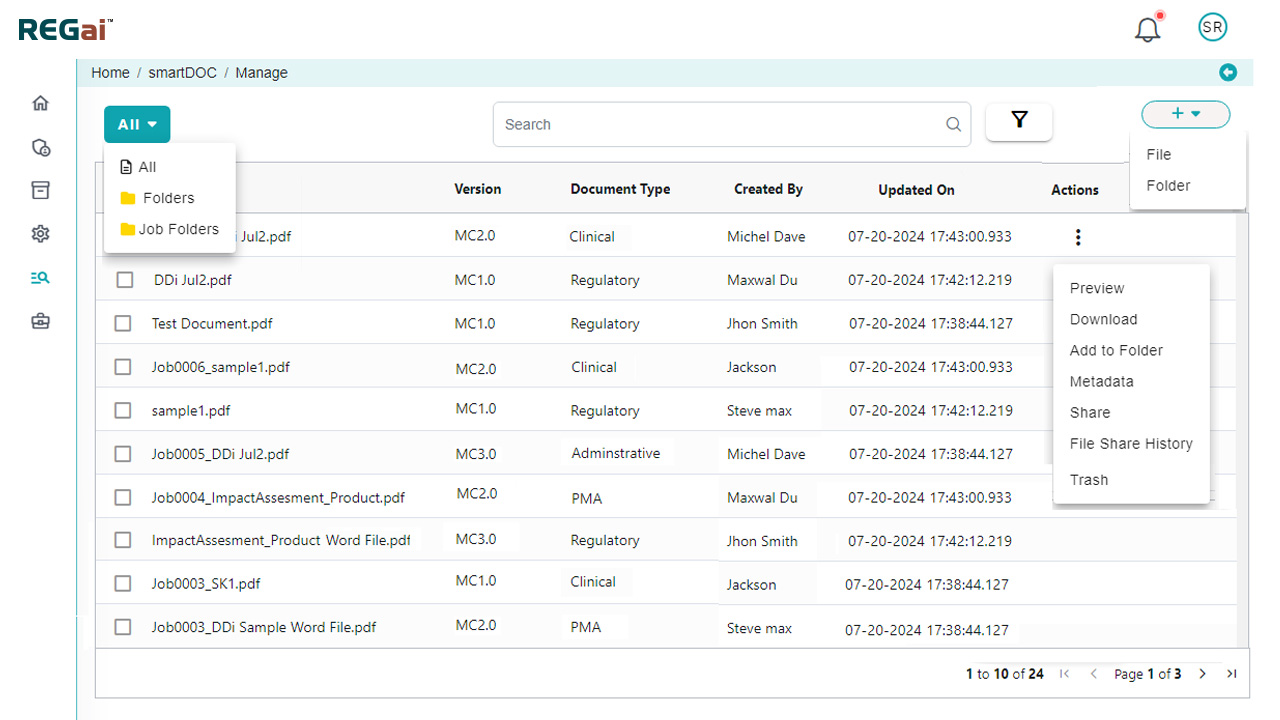
Pre-Publishing (Document level):
- Publishing Rules: Standard Out-of-box rules for FDA, EU, and other regions PLUS you can use any custom publishing rules from our vast library for MS Word and PDF (Tables, Font management, TOC, Bookmarks, Headers/Footers, Hyperlinks, File-level general options, and more)
- How to Use: 3 options: a) Word Plugins or b) Use our tool and upload files from your desktop/edms or c) we can connect to your existing Document stores and perform publishing as batch (hourly, daily or your schedule)
- Interfaces: With our pre-built interfaces, we can connect to your current eCTD systems, RIM, EDMS, ERP, SharePoint, Documentum, BOX, and other 3rd party Sources.
Post-Publishing:
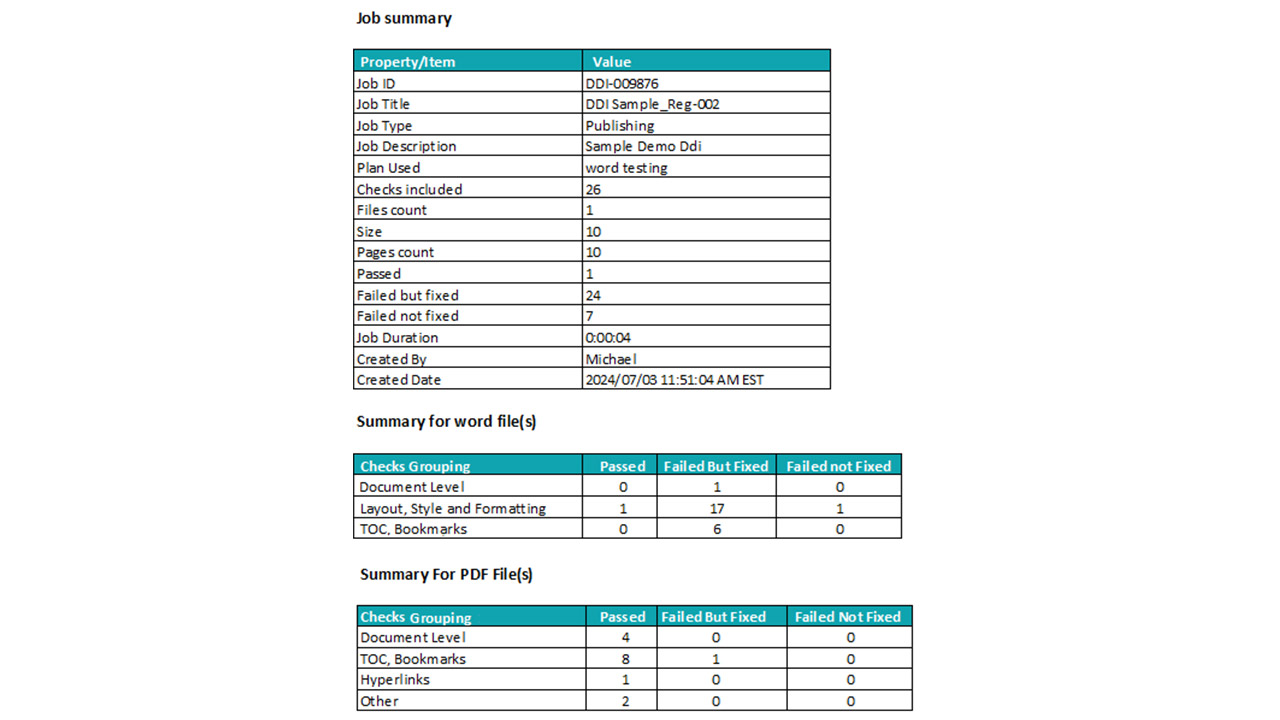
Automate validation with customized Dossier checks covering dossier elements, titles, and data/content level.
-
Automate validation based on your checklists
-
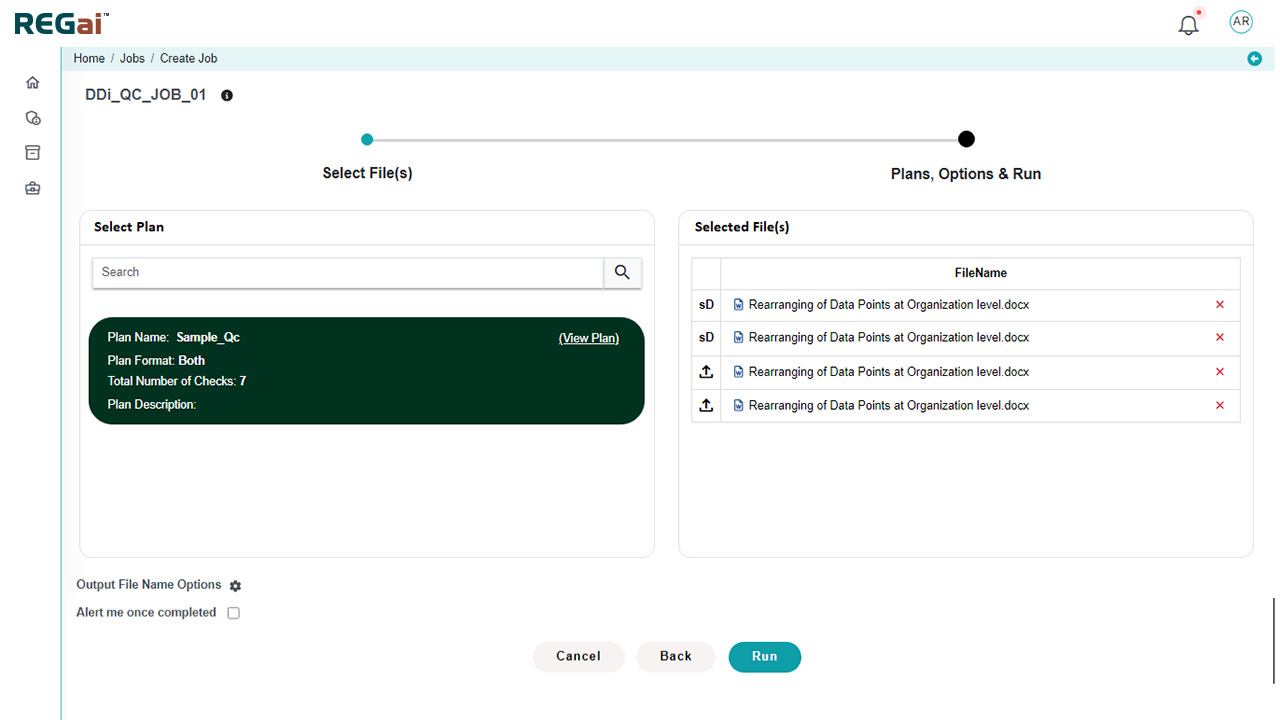
Create custom validation plans at dossier level and different plans for the documents within those dossiers (Standard and Custom dossiers)
-
Inter-Document hyperlinks check and automate updates
-
Auto-update of File Names, Elements, Leaf titles matching to your internal standards
-
Selectively test validation paths of the defined checks and parameters
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you


