
electronic
Instructions For Use (eIFU)
Full-Service Solution Provider
Managing electronic IFUs reduces print costs, manual efforts, and compliance issues. In addition, it’s mandatory for some regions countries. See how Visu eIFU can make this transition smoother, cost effective and ensuring compliance.
Why should you consider Visu eIFU:
- Certified in ISO 13485
- Single solution provider for both Software and Print service management
- Audited by multiple Notified Bodies till date
- Regulatory, Labeling and IT subject experts to guide the transition process
- Data in ISO 27001 certified data centers (USA/Germany) and GDPR compliant
- Trusted by many Manufacturers for their eIFU for several years
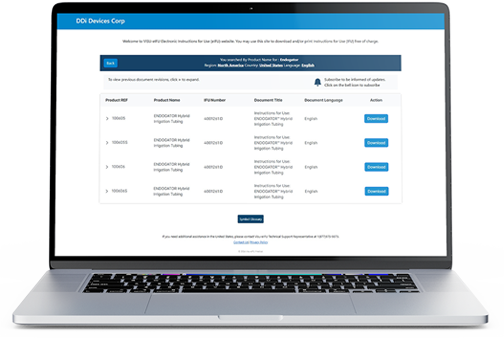
Made with Visu eIFU Software
Website / Portal (examples of What your end users see)
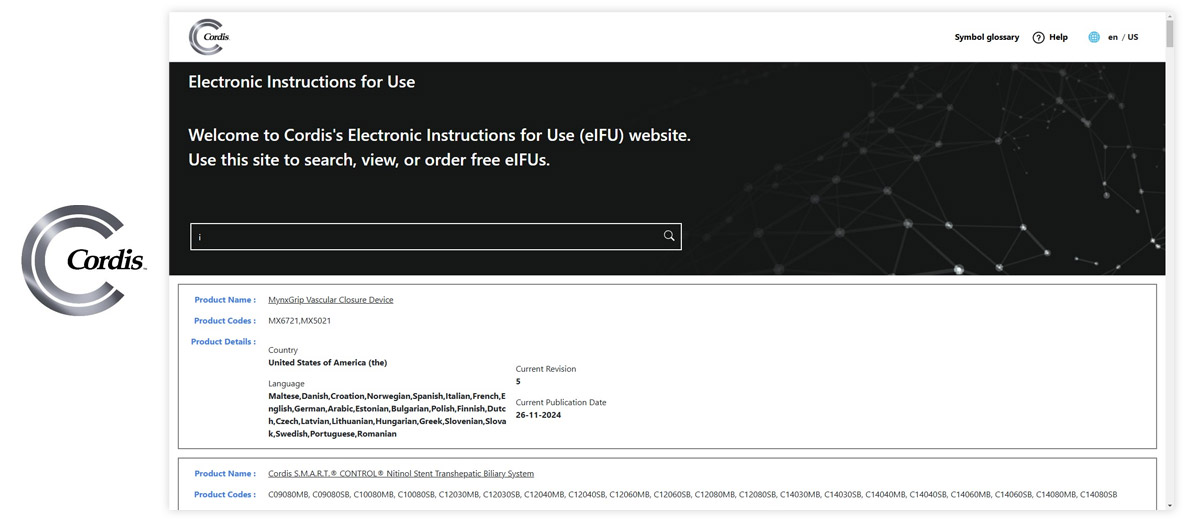
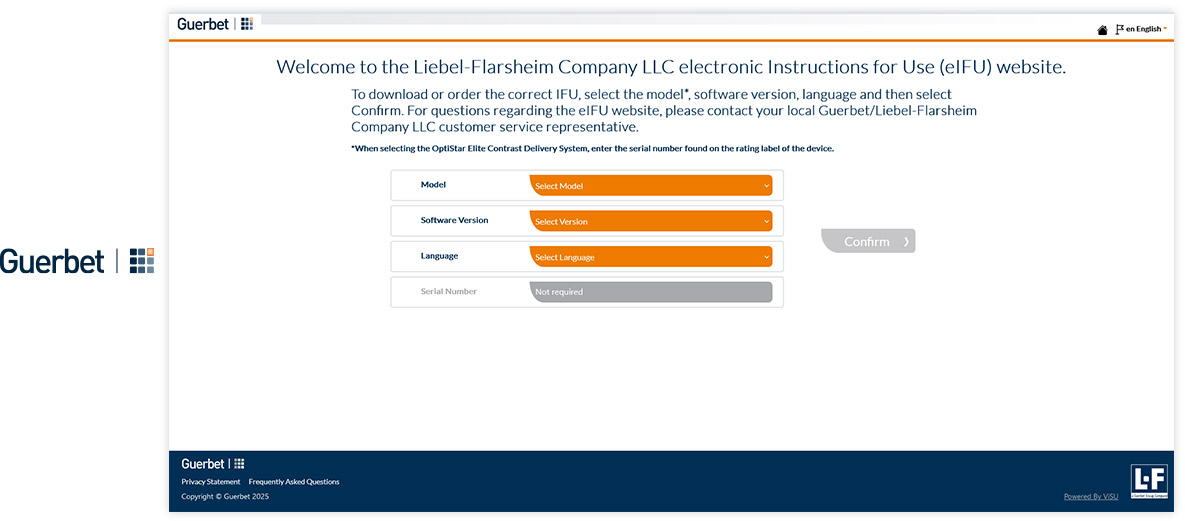
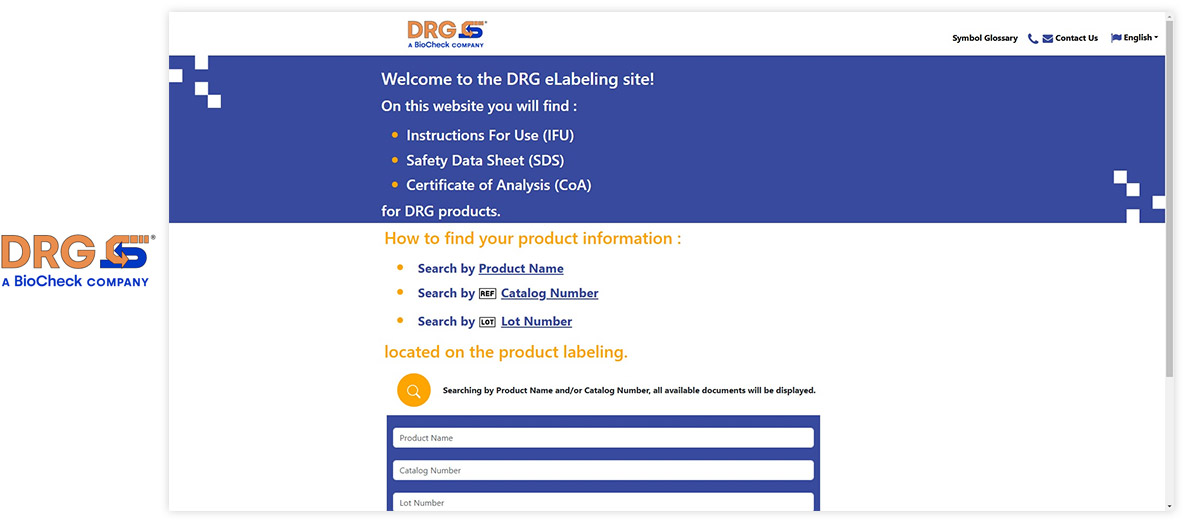
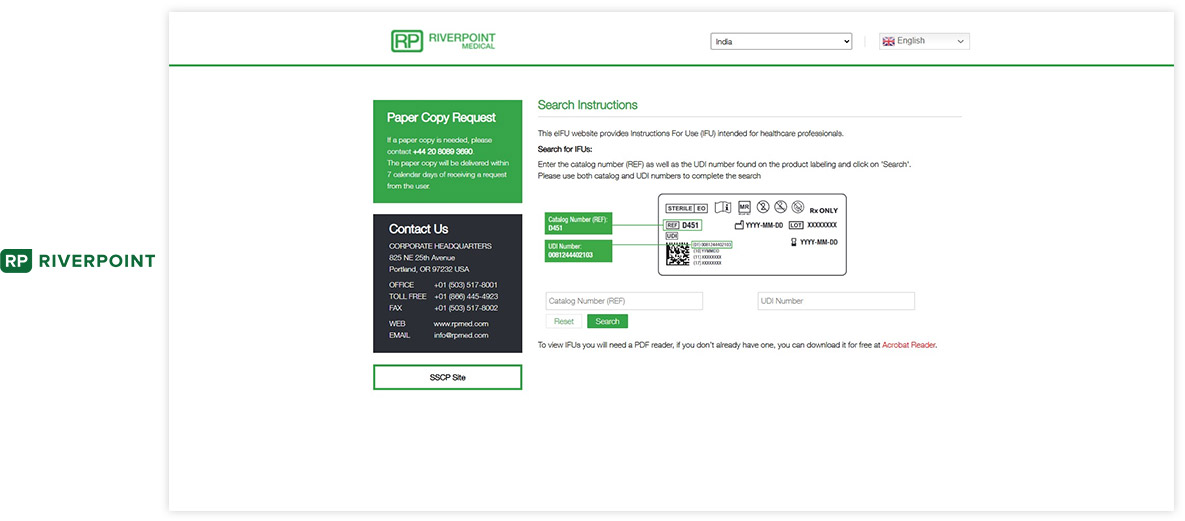
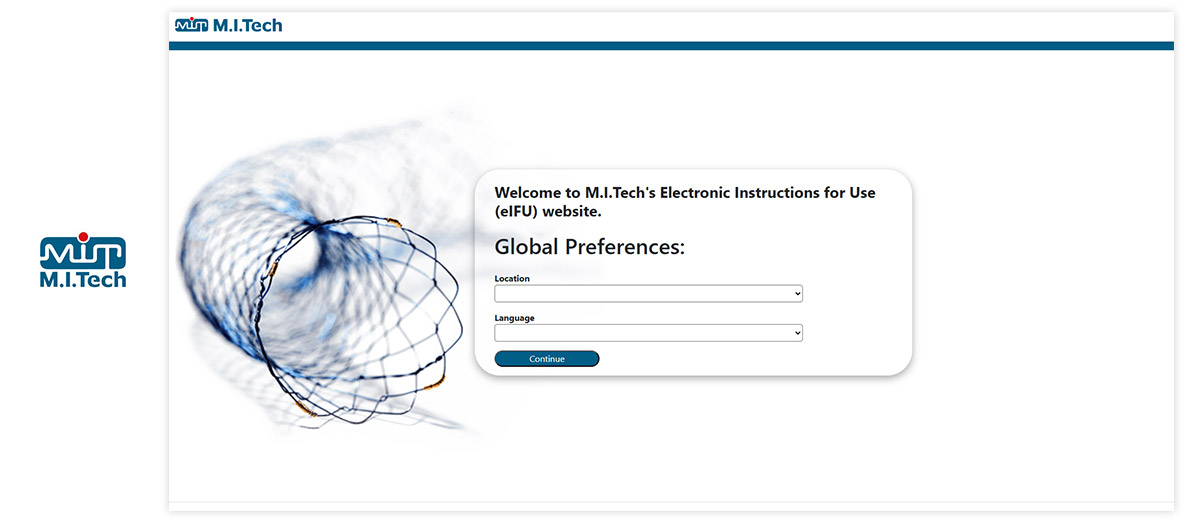
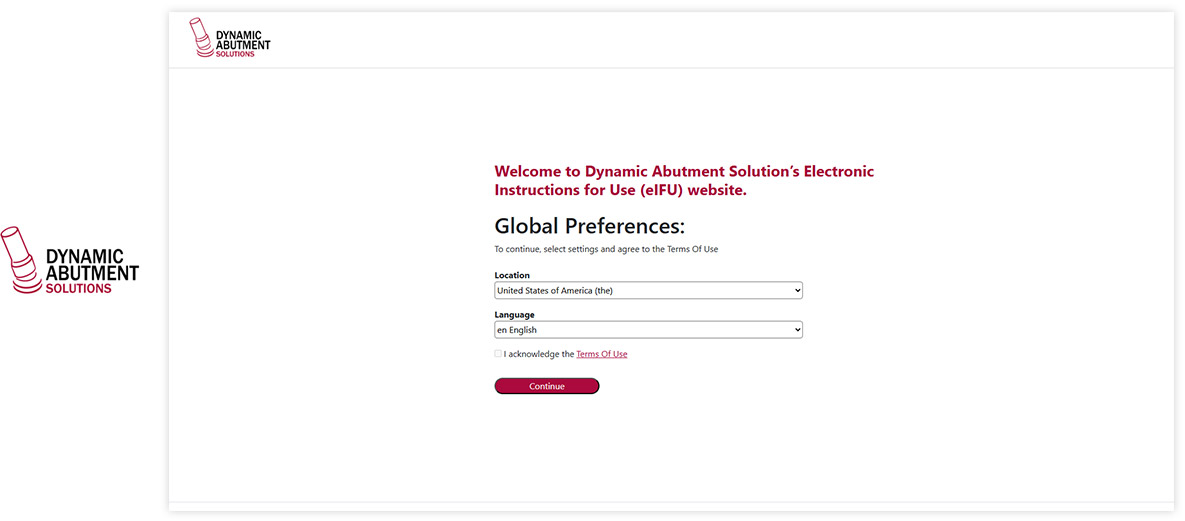
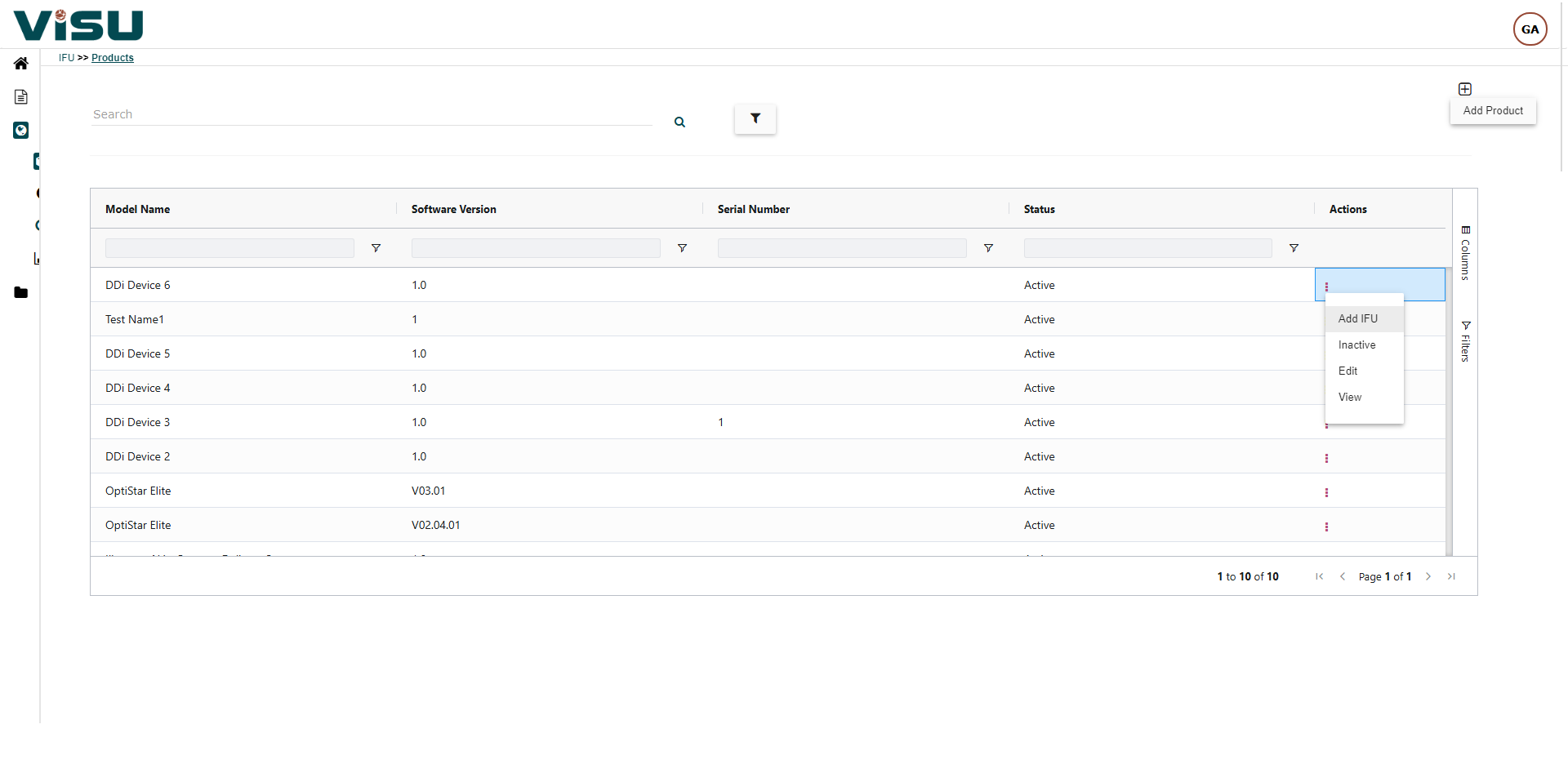
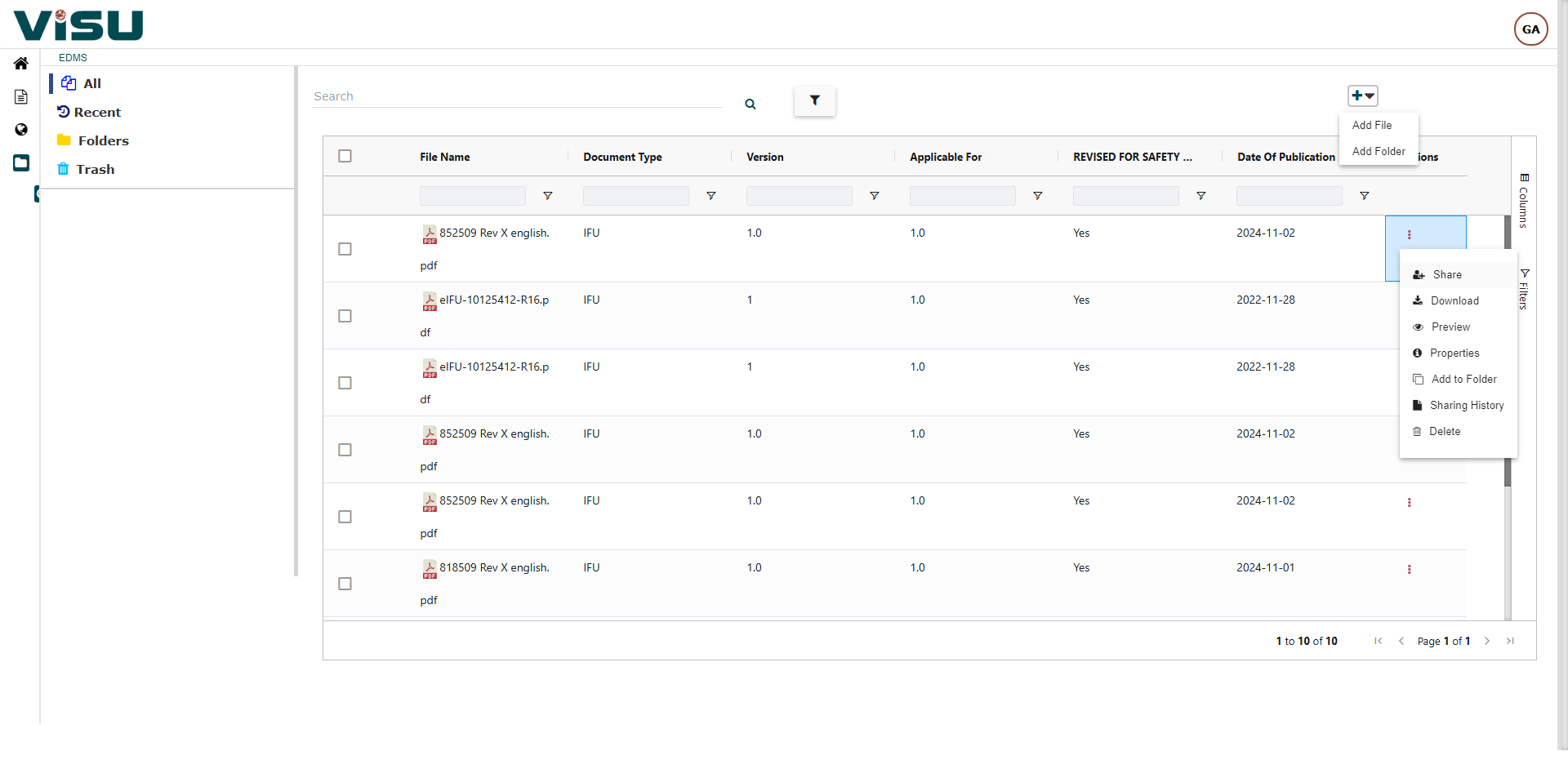
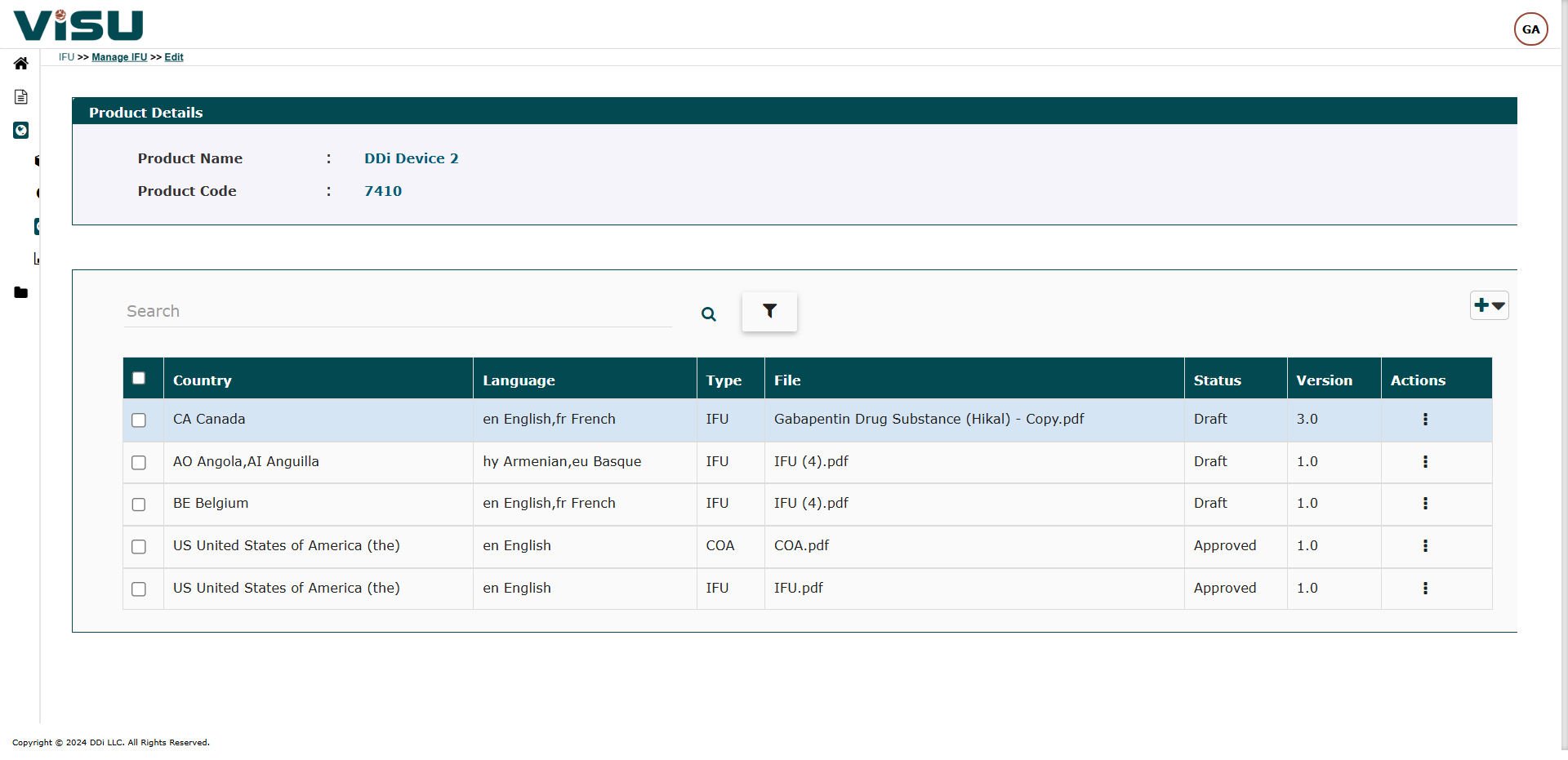
Visu eIFU Admin Access (from this you manage Data & Documents)
- MDR (EU 2021/2226), FDA and other regulations Compliant
- Role & Permission Driven
- Multi-language, Multi-versions linked by Products and Document versions
- Enterprise version option with workflows, connect to your PLM & EDMS, custom branding and other premium features
- 21 CFR Part 11, GAMP compliant and validated
- Security measures and Privacy Policy are in compliance with GDPR
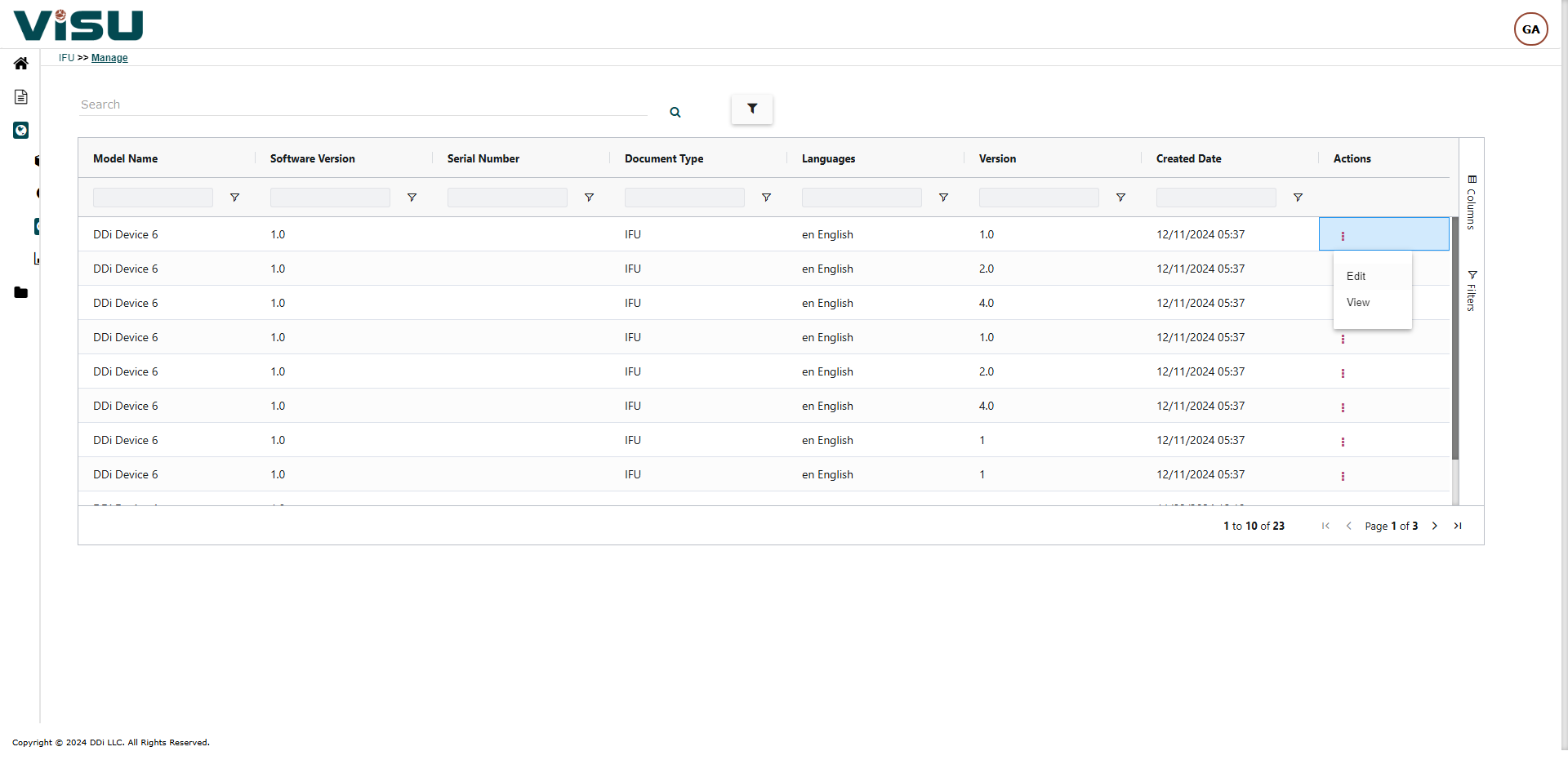
PRINT SERVICES
- Full time in-house Call Center that follows ISO 20000 policies
- Handle “print” requests and ship hard copies (or optionally, coordinate with manufacturer fulfillment teams if you want to keep this in-house)
- Maintain TAT for 7 days (as per EU Regulation)
- Provide audits trail reports for action by your users to help maintain traceability, and compliance with regulatory requirements.
We’re Here To Help
Get in touch with us
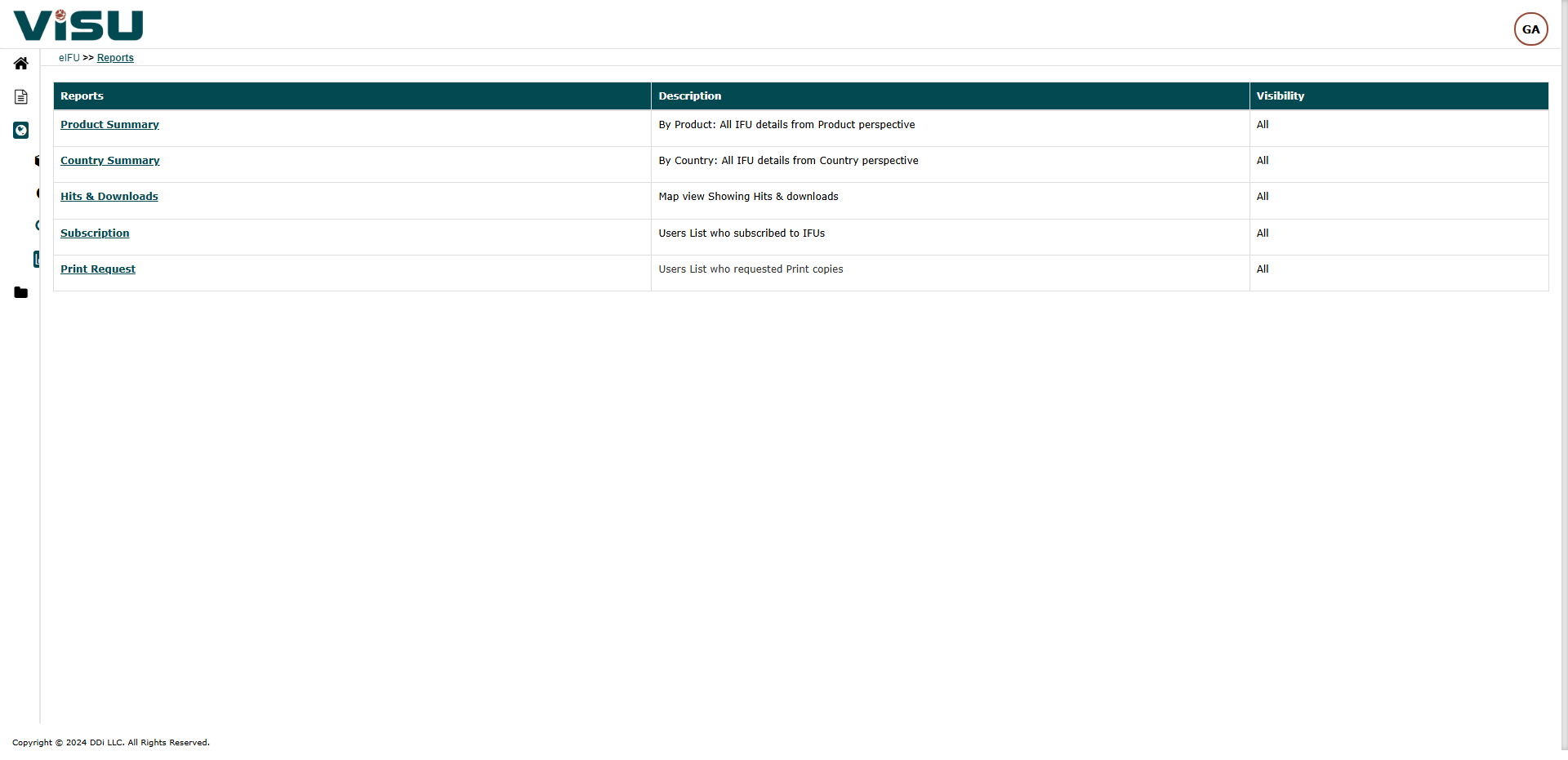
eIFU Regulations & Guidelines
-
Regulations and Guidelines
eIFU guidance for IVDs
eIFU regulation for MDs (under MDR)
eIFU regulation for MDs (under MDD)
EU regulation for IVDs
EU regulation for MDs
GDPR
MEDDEV 2.14/3 rev.1 Supply of Instructions For Use (IFU) and other information for In-vitro Diagnostic (IVD) Medical Devices.
Read more:Commission Implementing Regulation (EU) 2021/2226 of 14 December 2021 laying down rules for the application of Regulation (EU) 2017/745 of the European Parliament and of the Council as regards electronic instructions for use of medical devices.
Read more:Commission Regulation (EU) No 207/2012 of 9 March 2013 on electronic instructions for use of medical devices.
Read more:
Regulation (EU) 2017/746 of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU.
Read more:Regulation (EU) 2017/745 of 5 April 2017 on Medical Devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC.
Read more:Regulation (EU) 2016/679 of 25 May 2018 on Regulation on the protection of natural persons regarding the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (Data Protection Directive).
Read more: -
US Regulations (FDA)
FDA regulation for MDs & IVDs
FDA regulation for MDs & IVDs on labeling and advertisements
FDA regulation on use of electronic records & signatures
FDA regulation to authorize eIFU
Federal Food, Drug, and Cosmetic Act (FD&C Act) – Chapter V: Drugs and Devices – Part A Drugs and Devices and Part D Dissemination of Treatment Information.
Read more:U.S. Code, Title 21, Chapter 9, subchapter V, Part A, §352 Misbranded drugs and devices.
Read more:U.S. Code, Title 21, Chapter 11, Electronic records; electronic signatures.
Read more:Section 206 of the Medical Device User Fee and Modernization Act (MDUFMA) (New section 502(f) of the Federal Food, Drug, and Cosmetic Act) Electronic Labeling for Prescription Devices Intended for Use in Health Care Facilities.
Read more:US Guidance Documents (FDA)
FDA regulation for MDs & IVDs
Guidance for Industry and FDA Staff: Acceptable Media for Electronic Product User Manuals
Guidance for Industry; March 2006
Read more:March 2010.
Read more: -
Notice 10-123767-875: Guidance for the labelling of Medical Devices, not including in vitro diagnostic devices.
Read more -
Normative Instruction – IN No. 4, of June 15th 2012.
Read more -
SFDA/MDS Guidance on Requirements for Electronic Instructions for Use (e-IFU) of Medical Devices (MDS – G41, of September 29, 2019)
-
Electronic Instructions for Use – eIFU: For professional users of medical devices (including IVDs) – v1.0 August 2018 (Therapeutic Goods Administration).
Read more -
SDLC standard used for the development of the IFUcare website
IEC 62304:2006: Medical device software – Software life cycle processes
ISO 13485
Medical Devices - Quality Management Systems - Requirements for Regulatory Purposes.
ISO 14971
Medical Devices – Application of Risk Management to Medical Devices
Related Blogs
Let's talk about how DDi can help you
