Blogs
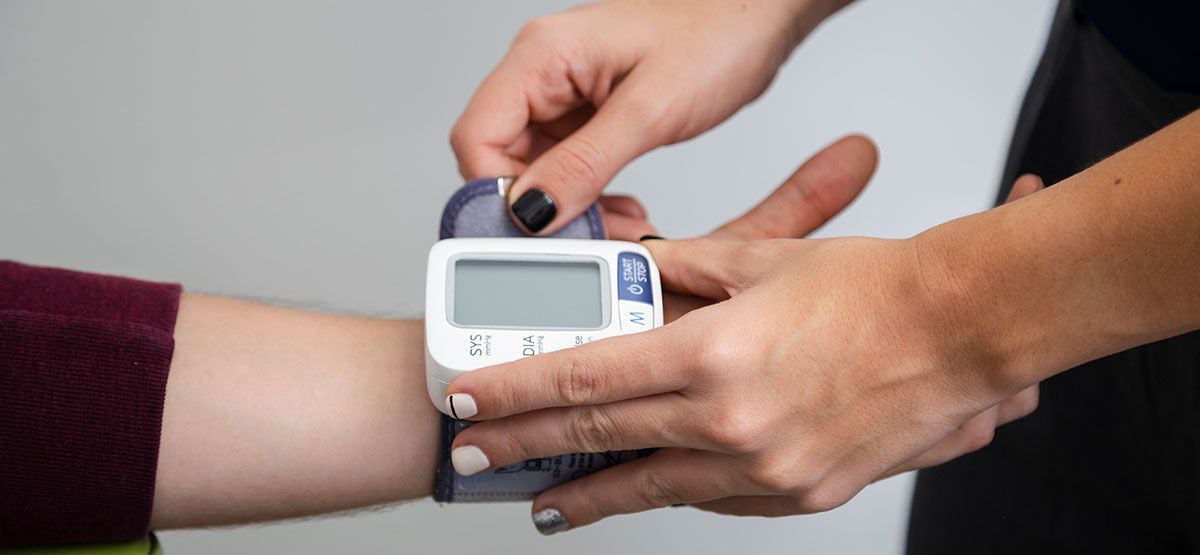
Measures to Overcome Low Stock Supplies in Clinical Trials Using IRT
Management of clinical supplies in clinical trials has become a major hurdle in this new era of clinical research. With the complex challenges that clinical trials shoot in terms of the design or …

Main Factors that Unify IRT in Clinical Supplies
IRT has created its footprint in the field of clinical research and has evolved drastically in such a way that it has bought a major difference in perspective of clinical trials over the decades. …
Get the latest updates from DDi
Explore Topics
- Automation & AI (5)
- Clinical Automation (8)
- Consumer Health (1)
- IRT & Clinical Supplies (21)
- Labeling (16)
- Regulations (23)
- Regulatory Automation (14)
- Regulatory Biopharma (2)
- Regulatory Content Management (5)
- Regulatory Information Management (21)
- UDI (11)
- Writing (12)
Most Used Tags
Artificial Intelligence (5)
Clinical Trials (17)
Clinical Trial Supplies Management (11)
Compliance Solutions (7)
Digital Transformation (4)
eIFU (5)
Electronic IFU (5)
EUDAMED (5)
EU MDR (4)
Healthcare (5)
Healthcare Compliance (6)
Healthcare Innovation (9)
Healthcare Technology (4)
Health Tech (4)
Labeling Automation (5)
Medical Device Compliance (7)
Medical device Labeling (4)
Medical Device Regulation (6)
Medical Devices (18)
Medical Writing Automation (4)
Medtech (6)
Patient Safety (5)
REGai (10)
Regulatory Affairs (9)
Regulatory Automation (13)
Regulatory Compliance (26)
Regulatory Information Management (7)
Regulatory Submissions (4)
UDI (4)
UDI Compliance (5)
CONNECT WITH US

The First Step
Let's talk about how DDi can help you