Regulatory Project Management & Tracking
for Pharma Biotech
Transforming Regulatory Execution with AI-Powered Project Management
In the complex and compliance-heavy world of pharma and biotech, regulatory project management (RPM) is pivotal to product success. From clinical trial approvals to global submissions and post-market surveillance, companies face increasing demands for speed, precision, and coordination.
DDi’s regulatory project management platform empowers teams to automate, track, and scale regulatory operations with AI-driven intelligence – delivering faster approvals, reduced risk, and greater efficiency across global markets.
Challenges in Traditional Regulatory Project Management
Despite their critical role, many regulatory affairs teams still operate with outdated tools – manual trackers, static spreadsheets, fragmented emails. These methods are error-prone, inefficient, and ill-equipped to handle the complexities of modern drug development.
Common pain points include:
- Disconnected data across teams and geographies
- Human errors in submission timelines and documentation
- Limited visibility into bottlenecks and task dependencies
- Manual monitoring of evolving global regulations
The result? Missed deadlines, higher compliance risk, and costly delays.
What Makes DDi’s AI-Driven RPM Solution Different
We go beyond traditional project management by integrating automation, intelligence, and real-time regulatory awareness into every step of the workflow.
AI-Enabled Submission Tracking
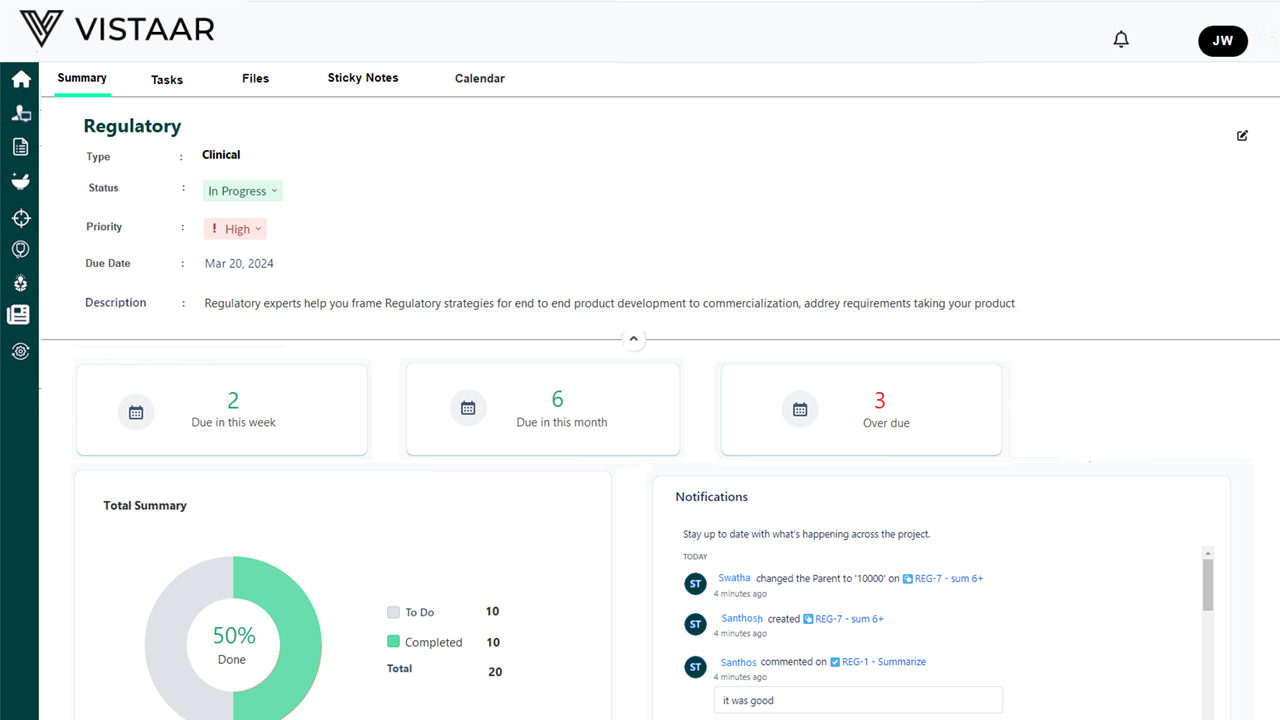
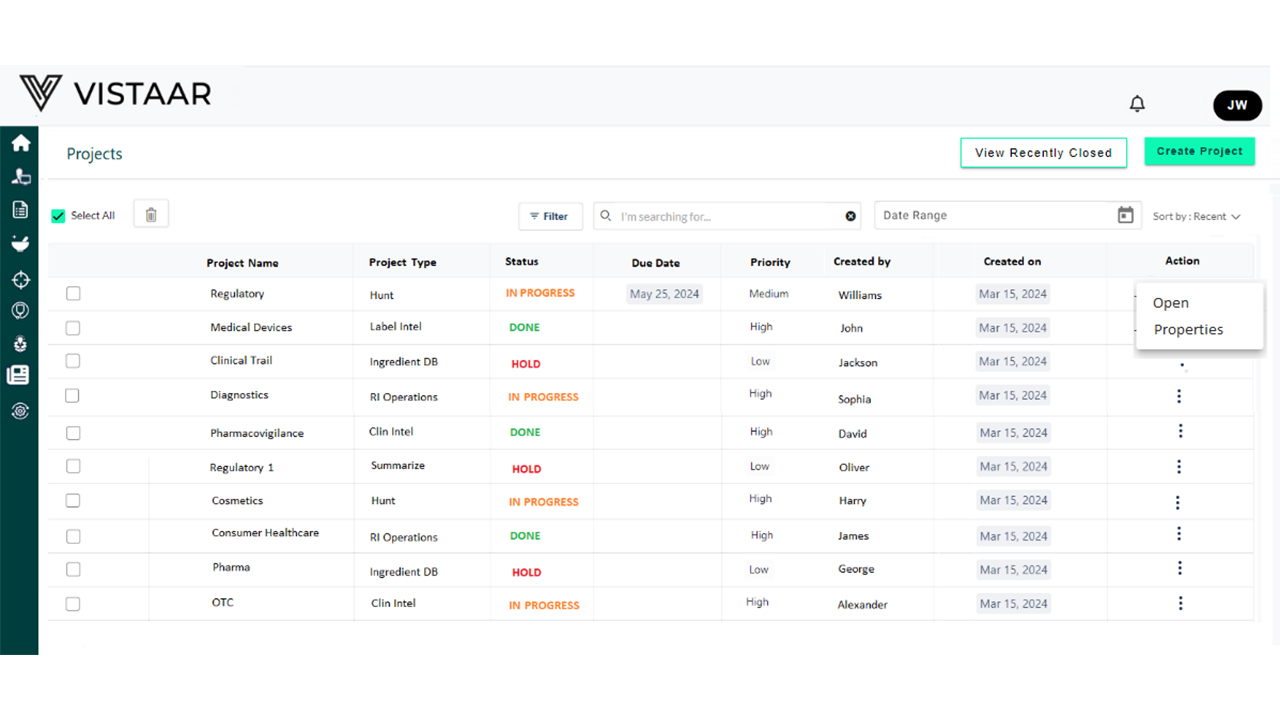
Track every milestone – IND, NDA, BLA, variations – automatically. Smart Gantt charts, dynamic timelines, and regulatory dependencies help you stay on schedule and in control.
Automated Document Intelligence
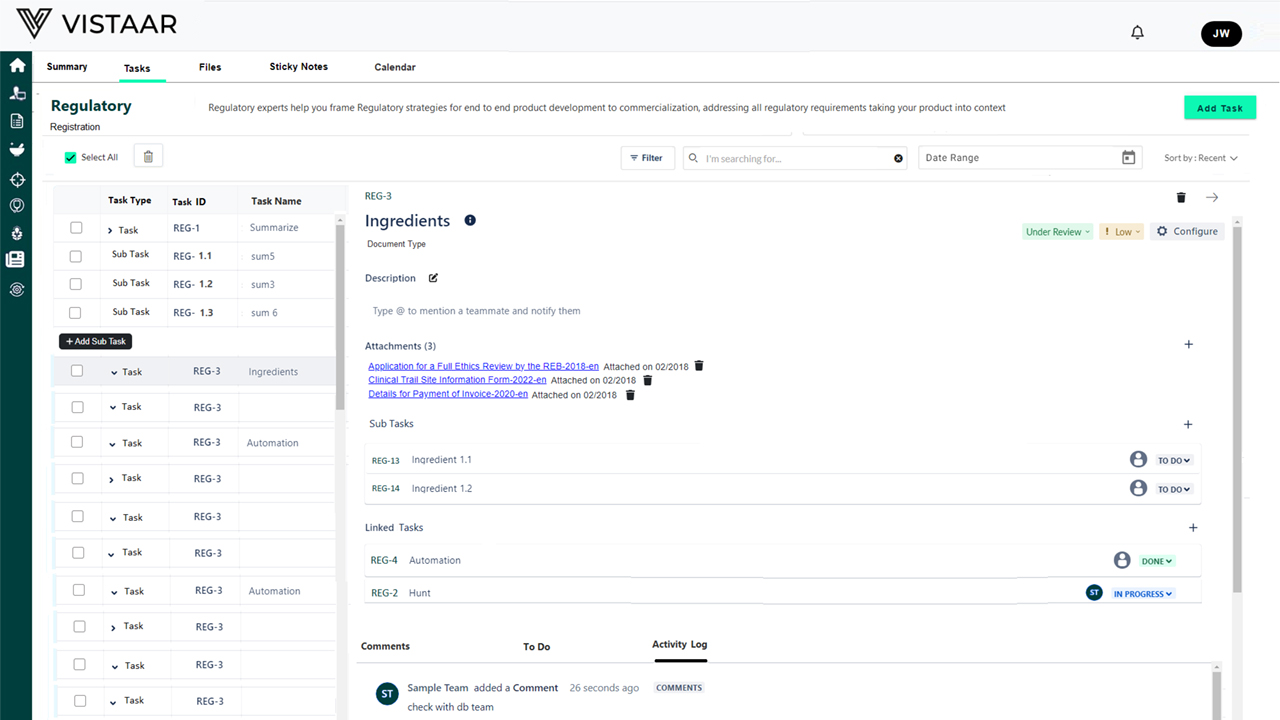
Use AI to extract, validate, and classify regulatory content across formats like CTDs, CSRs, and protocol amendments. Auto-tagging, version control, and NLP-based reviews ensure precision and compliance.
Real-Time Alerts & Regulatory Intelligence
Stay ahead of global authority updates with automated scanning of FDA, EMA, PMDA, and MHRA websites. Automatically update your internal checklists and project plans accordingly.
Predictive Risk Management
AI models analyze historical submission data to flag risks, forecast approval timelines, and identify potential regulatory reviewer queries.
Cross-Functional Collaboration Portals
Role-based dashboards give R&D, clinical, QA, legal, and regulatory teams a single source of truth. Enable faster decisions and cleaner communication.
We go beyond traditional project management by integrating automation, intelligence, and real-time regulatory awareness into every step of the workflow.
AI-Enabled Submission Tracking
Track every milestone – IND, NDA, BLA, variations – automatically. Smart Gantt charts, dynamic timelines, and regulatory dependencies help you stay on schedule and in control.
Automated Document Intelligence
Use AI to extract, validate, and classify regulatory content across formats like CTDs, CSRs, and protocol amendments. Auto-tagging, version control, and NLP-based reviews ensure precision and compliance.
Real-Time Alerts & Regulatory Intelligence
Stay ahead of global authority updates with automated scanning of FDA, EMA, PMDA, and MHRA websites. Automatically update your internal checklists and project plans accordingly.
Predictive Risk Management
AI models analyze historical submission data to flag risks, forecast approval timelines, and identify potential regulatory reviewer queries.
Cross-Functional Collaboration Portals
Role-based dashboards give R&D, clinical, QA, legal, and regulatory teams a single source of truth. Enable faster decisions and cleaner communication.
Key Benefits of Using DDi for Regulatory Project Management
- Faster Regulatory Approvals – Automated submissions, fewer errors, smoother workflows
- Audit-Ready Compliance – Complete version control, traceability, and documentation logs
- Reduced Manual Work – Eliminate repetitive, low-value tasks
- Scalable Across Global Submissions – Manage multiple products and regions in parallel
- Actionable Insights – Data-driven decisions through predictive dashboards
Built-In AI Capabilities That Power Success
- Natural Language Processing (NLP): Understands and classifies complex regulatory content
- Machine Learning (ML): Learns from past projects to optimize future timelines
- Predictive Analytics: Anticipates delays, queries, and workload bottlenecks
- Agentic AI Models: Simulate expert reasoning for automated decision support
The Future of Regulatory Project Management Is Intelligent
AI isn’t just streamlining processes - it’s becoming a strategic partner in regulatory success. Expect innovations such as:
- Voice-activated regulatory assistants
- Self-updating compliance platforms
- AI-powered submission guidance in real time
- Integrated post-market surveillance monitoring
DDi helps you stay ahead - not just with tools, but with transformative capability.
Ready to Automate Your Regulatory Workflow?
Whether you're planning your next IND, preparing for global filings, or managing cross-functional compliance, DDi's AI-powered platform ensures you do it faster, better, and smarter.
Schedule a Demo or connect with our Regulatory Automation experts today
Let's talk about how DDi can help you


