Regulatory Intelligence & Solutions
Global requirements change regularly. To read or analyze larger documents, translating non-English regulations is a time consuming process. In addition, some countries do not provide detailed regulations and interpreting such short documents need deeper analyzing capacity. Though there is no shortage of updates and documents in various public domains, getting accurate updates faster from authentic sources is the key.
To accomplish this, we include these 3 areas:
-
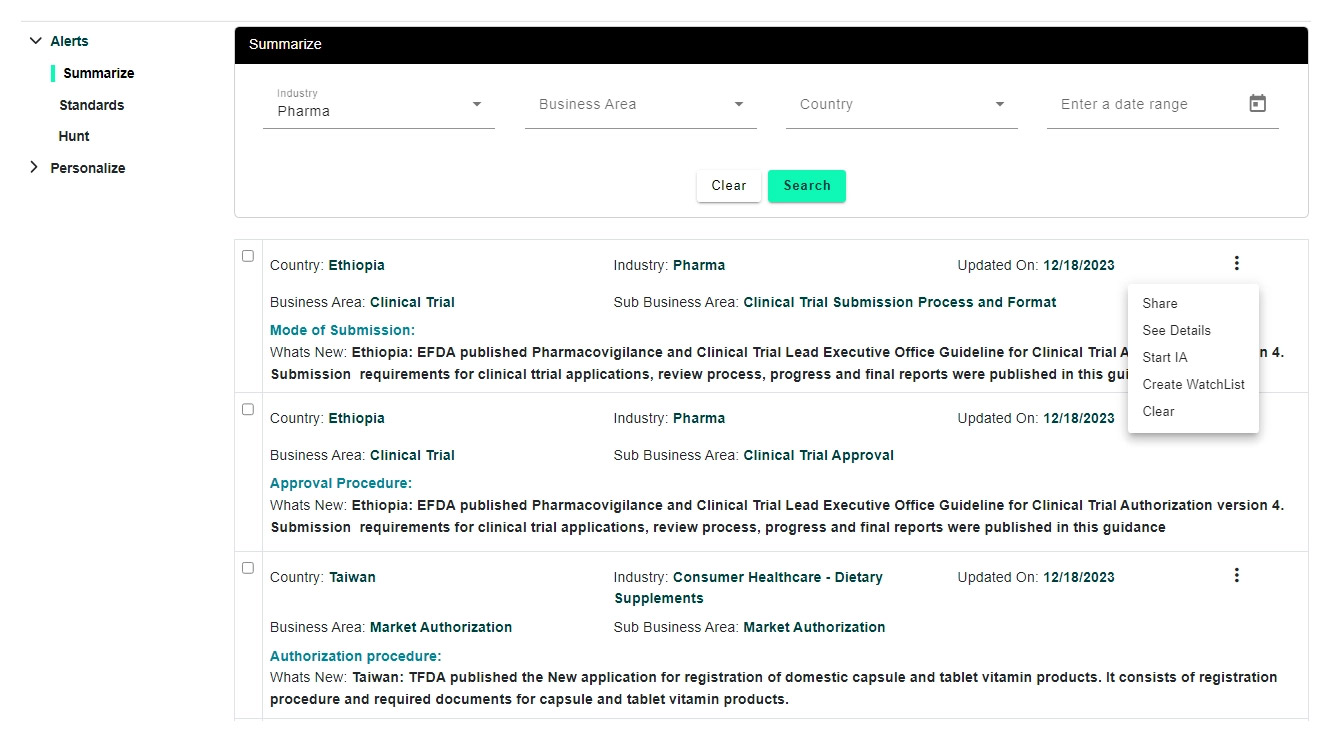
Regulatory monitoring (you can opt for alerts that are manually vetted by our reg analysts based on industry, functional area and set a frequency on how often you prefer to receive) and share (individual alerts or group few and convert to a newsletter and share to other stake holders using built-in newsletter functionality)
-
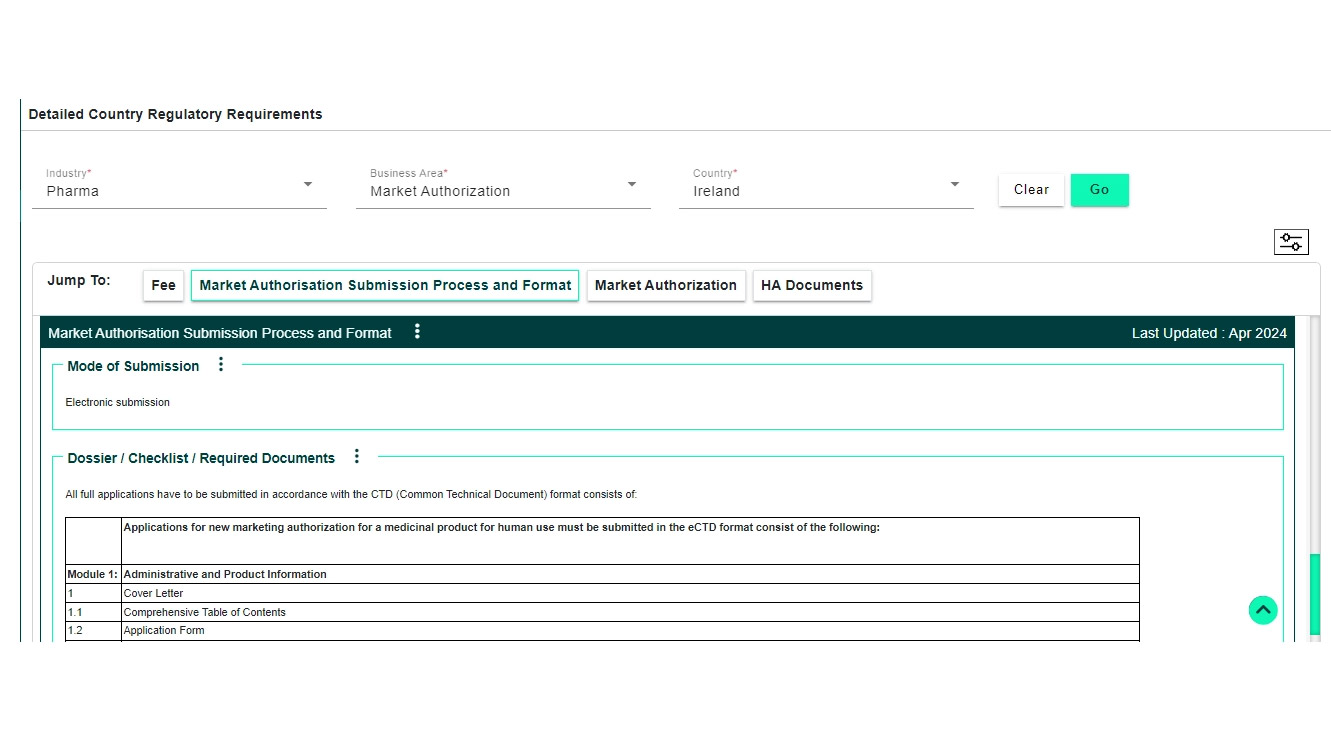
Global Regulatory Requirements Database (covers different business/functional areas including clinical, IRB, market launches, CMC, GMP standards, submission checklists, fees, labeling, import/export, marketing, safety/PV, renewals/variations and life cycle requirements)
-
Regulation & Guidance documents repository search
1
2
We’re Here To Help
Get in touch with us