Regulatory Strategy
Regulatory strategy is often a formal plan that aligns regulatory activities to business strategy, so as to bring a new or modified product to market. Formulating this plan would require your consideration of various regulatory issues in the target markets you wish to place your product. When well planned, your regulatory strategy should be balanced, realistic, achievable, and in support of your organization’s goals. It identifies important regulatory requirements to be addressed and provides overall definition and clear direction for your product development team, even outlining the reasons for the path to be taken.
To accomplish this, we include these 4 areas:
-
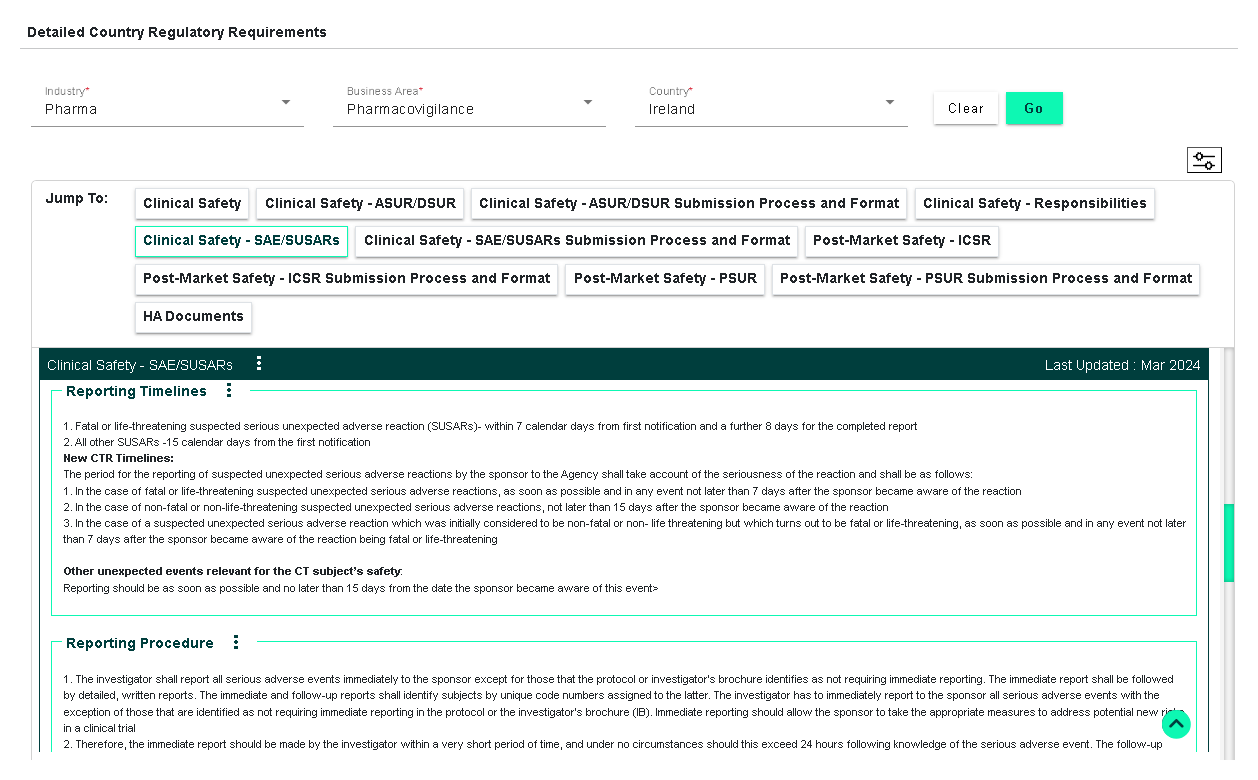
Global Regulatory Requirements Database (covers different business/functional areas including clinical, IRB, market launches, CMC, GMP standards, submission checklists, fees, labeling, import/export, marketing, safety/PV, renewals/variations and life cycle requirements)
-
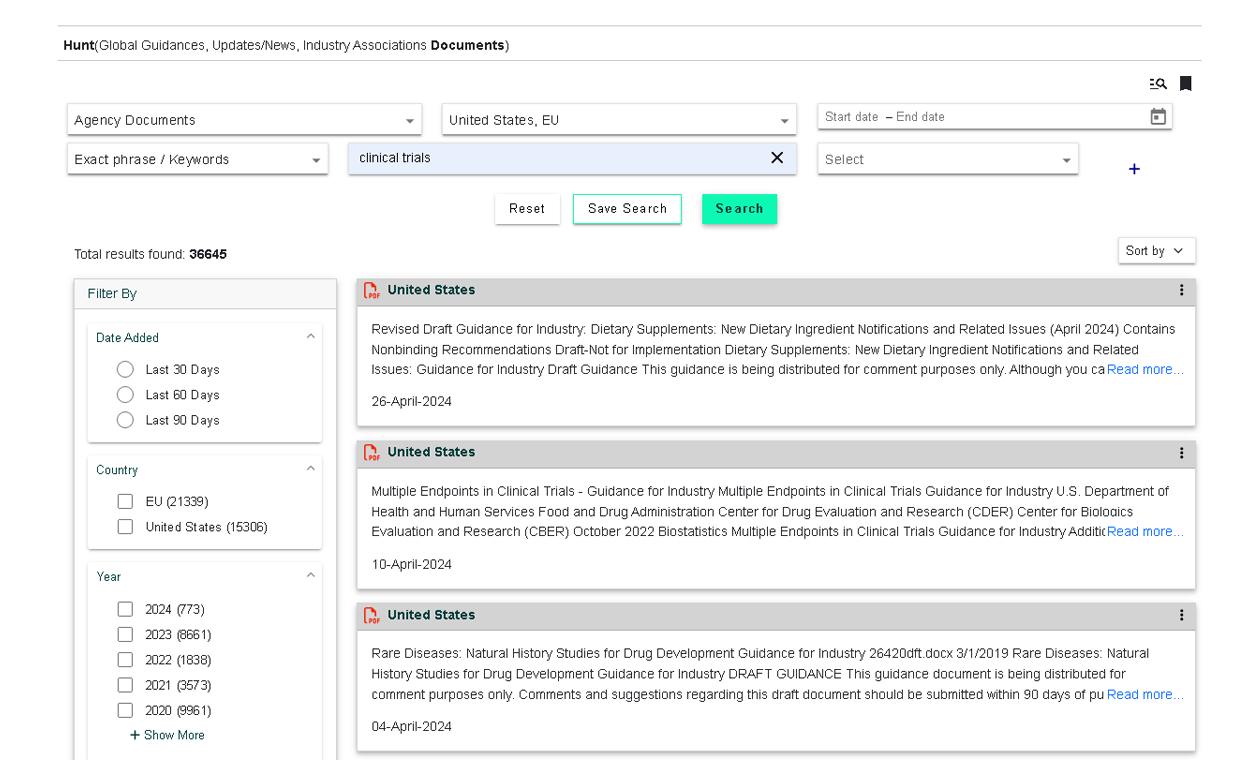
Regulation & Guidance documents repository search
-
Regulatory document creation (you can use your templates), collaborative review (both with internal and external stakeholders, while users who do not need to have login access to this system), auto-format (system will do all document formatting from fonts to tables to headers/footers etc)
-
Project creation (for long-term or large projects you can create tasks, assign to team, track – full online project module included)
1
2
We’re Here To Help
Get in touch with us