Interactive Response Technology System: The Backbone of Modern Clinical Trials
Clinical trials today demand speed, accuracy, and compliance. Managing randomization, drug supply, and patient enrollment manually is no longer sustainable. That’s where the Interactive Response Technology System comes in a powerful automation platform that simplifies complex trial operations while ensuring accuracy, compliance, and real-time visibility. This robust Interactive Response Technology System is essential for modern research.
What Is an Interactive Response Technology (IRT) System?
An Interactive Response Technology System is a digital solution used in clinical trials to manage randomization, drug supply, subject tracking, and site operations. It allows sponsors, CROs, and sites to control critical trial parameters through automated workflows, accessible via web or voice interfaces. The use of an IRT system is fundamental to IRT in clinical trials for maintaining protocol integrity.
IRT systems are designed to ensure that each patient receives the right treatment at the right time and that clinical supplies are managed efficiently across global sites. By automating manual processes, the IRT system helps maintain blinding integrity and ensures data accuracy throughout the trial lifecycle. This is a core function of IRT in clinical trials.
In essence, the IRT system is the operational control center of a clinical study, bridging participants, investigators, and supply teams under one secure and compliant environment.
Key Features of an Interactive Response Technology System
Modern IRT systems come packed with advanced capabilities designed for scalability and precision. Some of the most essential features include:
Randomization and Enrollment Management
The clinical trial randomization system within the IRT automates patient randomization based on study protocols, ensuring unbiased treatment allocation.
Drug Supply and Inventory Control
The system, functioning as sophisticated clinical supply management software, tracks medication shipments, site inventory, and re-supply triggers in real time.
Real-Time Data Access
Sponsors and CROs can view live reports on patient enrollment, supply levels, and site performance, enabling proactive decision-making.
Integration and Scalability
IRT integration with EDC and CTMS is seamless, ensuring a unified data ecosystem across the clinical technology stack.
Compliance and Audit Trails
Comprehensive audit logs, 21 CFR Part 11 compliance, and role-based access ensure regulatory adherence and data security for the IRT system.
How an IRT System Works: Step-by-Step Process
An Interactive Response Technology System follows a well-defined operational workflow that supports each stage of a clinical trial. Below is an overview of the typical process:
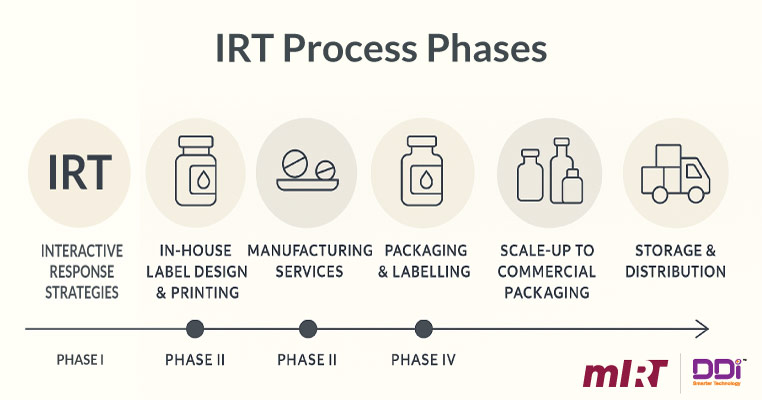
Study Setup
Before the trial begins, the sponsor or CRO configures study parameters such as treatment arms and site hierarchies in the IRT system.
Patient Enrollment
Investigators register subjects into the clinical trial randomization system. The IRT verifies eligibility criteria and assigns a randomization code.
Treatment Allocation
Based on the randomization logic, the IRT system determines which treatment each participant should receive.
Supply Management
The clinical supply management software monitors site inventory, predicts future requirements, and automatically triggers re-supply requests.
Data Monitoring and Reporting
Real-time dashboards provide oversight into subject progress, shipment status, and protocol deviations.
By automating these steps, the IRT system eliminates manual intervention, reduces human error, and ensures faster trial execution.
Benefits of Using Interactive Response Technology in Clinical Trials
Implementing an IRT system offers several measurable advantages for sponsors and research teams:
Enhanced Accuracy
Automation in the clinical trial randomization system minimizes the risk of allocation errors.
Improved Compliance
Built-in validation rules and audit trails in the IRT system support compliance with GCP, FDA, and EMA regulations.
Streamlined Supply Chain
Predictive algorithms in the clinical supply management software optimize drug supply, reducing waste.
Real-Time Visibility
Instant access to enrollment and inventory data from the IRT system helps study teams make informed decisions.
Reduced Operational Costs
By automating routine tasks, the IRT system lowers administrative burden and accelerates timelines.
Better Patient Safety
Accurate tracking of supplies and dosing by the IRT system ensures participant safety.
IRT vs IVRS vs IWRS: Understanding the Differences
Although terms like IRT, IVRS, and IWRS are often used interchangeably, there are subtle differences worth noting. The modern Interactive Response Technology System typically encompasses both.
IVRS (Interactive Voice Response System):
Uses a phone-based interface allowing users to interact with the system via voice or keypad inputs. Ideal for areas with limited internet connectivity.
IWRS (Interactive Web Response System):
A web-based version of an IRT system accessed through browsers, offering a more intuitive and data-rich interface.
IRT (Interactive Response Technology):
A broader term encompassing both IVRS and IWRS, representing the modern, unified approach combining both voice and web interactions.
The Role of IRT in Clinical Supply Chain Management
An efficient clinical supply chain is essential for successful trial execution. The IRT system plays a pivotal role by:
- Managing drug forecasting and supply allocation
- Tracking temperature-sensitive shipments
- Automating site re-supply and return logistics
- Maintaining blinding during drug assignment
By enabling end-to-end visibility and control, the clinical supply management software ensures that the right drug reaches the right patient at the right time.
Regulatory Compliance and Data Security in IRT Systems
IRT systems are built to meet stringent regulatory and data protection standards that govern clinical research:
- 21 CFR Part 11 and GxP compliance: Ensures electronic records from the IRT system are trustworthy.
- GDPR and HIPAA adherence: Protects patient data confidentiality within the IRT system.
- Comprehensive audit trails: Tracks every action within the IRT system for full traceability.
- Role-based access controls: Limits user access to the IRT system based on permissions.
Integration of IRT Systems with EDC and CTMS Platforms
A modern IRT system doesn’t operate in isolation it integrates with other key systems. Successful IRT integration with EDC and CTMS is crucial for a unified data ecosystem.
- EDC (Electronic Data Capture): Synchronizes subject and dosing data for seamless reporting via IRT integration with EDC and CTMS.
- CTMS (Clinical Trial Management System): Shares site, visit, and enrollment data for centralized monitoring.
- eCOA/ePRO: Links patient-reported outcomes with treatment and randomization data from the IRT system.
This integrated digital ecosystem, powered by robust IRT integration with EDC and CTMS, eliminates data silos, ensuring study efficiency.
Why Choosing the Right Interactive Response Technology (IRT) Vendor Matters
Selecting the right Interactive Response Technology (IRT) system provider is critical to the success of any clinical trial. With DDiSmart’s advanced IRT system, sponsors and CROs can ensure smarter trial management, greater flexibility, and faster execution all while maintaining global compliance.
Here are the key factors that make DDiSmart the preferred IRT partner for life sciences organizations worldwide:
1. Experience in Global Clinical Trials
DDiSmart brings deep expertise in deploying IRT solutions across global, multi-country clinical trials. Our proven track record ensures seamless management of randomization, drug supply, and site logistics all in compliance with diverse regulatory frameworks.
2. System Flexibility
The DDiSmart IRT platform is built for flexibility. It can be fully configured to meet the most complex study protocols, including adaptive designs, cohort-based randomization, and intricate supply chains. This ensures that your IRT solution aligns perfectly with your study’s scientific and operational goals.
3. Scalability
Whether you’re running a small Phase I trial or a large global Phase III program, DDiSmart’s IRT system scales effortlessly. Our architecture supports expansion across multiple geographies and therapeutic areas without compromising performance or compliance.
4. Integration Capabilities
DDiSmart IRT integrates smoothly with leading EDC (Electronic Data Capture) and CTMS (Clinical Trial Management Systems) platforms. This unified data environment enhances visibility, eliminates duplication, and accelerates decision-making across the clinical trial lifecycle.
5. Regulatory Compliance
Built on industry best practices, DDiSmart IRT complies with GxP standards and 21 CFR Part 11 requirements. The system ensures full auditability, data integrity, and security empowering sponsors to maintain confidence during regulatory inspections and submissions.
6. Customer Support and Training
With DDiSmart, you get more than just technology you gain a dedicated global partner. Our 24/7 support team and comprehensive user training ensure smooth implementation, reduced downtime, and faster adoption by study teams worldwide.
Why Choose DDiSmart as Your IRT Partner
By choosing DDiSmart, you align with a vendor that combines global trial experience, system flexibility, scalability, regulatory compliance, and exceptional customer support. Our intelligent automation and integration-driven approach make clinical supply and randomization management simpler, faster, and more reliable.
Empower your next clinical trial with DDiSmart’s Interactive Response Technology where innovation meets compliance, and efficiency drives success.
Future Trends in Interactive Response Technology Systems
The evolution of the Interactive Response Technology System continues as new technologies shape the future of clinical operations. Key trends include:
Artificial Intelligence (AI) and Predictive Analytics
AI-driven forecasting in the IRT system will further optimize supply chain management.
Cloud-Based and Decentralized Trials
Cloud-enabled IRT systems support remote site access and hybrid trial models.
Integration with Wearables and IoT Devices
Future IRT systems may connect directly to patient devices for real-time adherence tracking.
Advanced Data Visualization
Next-generation dashboards in the IRT system will use interactive visual analytics.
Continuous Compliance Automation
Automated validation tools in the IRT system will simplify global compliance.
Conclusion
An Interactive Response Technology System is no longer just a support tool it’s the digital backbone of modern clinical research. By automating randomization via a clinical trial randomization system and managing supplies with clinical supply management software, the IRT enhances accuracy and efficiency. Furthermore, seamless IRT integration with EDC and CTMS creates a powerful, unified clinical technology ecosystem. As the industry evolves, the Interactive Response Technology System will continue to be a pivotal asset in bringing innovative therapies to patients faster.
Get the latest updates from DDi
Explore Topics
- Automation & AI (16)
- Clinical Automation (9)
- Consumer Health (1)
- IRT & Clinical Supplies (24)
- Labeling (16)
- Regulations (26)
- Regulatory Automation (14)
- Regulatory Biopharma (3)
- Regulatory Content Management (5)
- Regulatory Information Management (23)
- UDI (11)
- Writing (12)
Recent Blogs
 Interactive Response Technolog…IRT & Clinical Supplies
Interactive Response Technolog…IRT & Clinical Supplies The Growing Complexity of Life…Automation & AI
The Growing Complexity of Life…Automation & AI BioPharma Regulatory Project M…Automation & AI
BioPharma Regulatory Project M…Automation & AI
Previous Post
Next Post
Related Posts
CONNECT WITH US

Let's talk about how DDi can help you