Clinical Project Management & Tracking
for Pharma & Biotech
Driving Faster, Smarter Clinical Execution with Intelligent Tracking
Clinical project management today involves far more than maintaining timelines and budgets. Pharma and biotech organizations must coordinate global stakeholders, manage regulatory expectations, mitigate operational risks, and ensure patient safety – all while accelerating development timelines. Yet many teams still rely on spreadsheets, static Gantt charts, and email updates, making it difficult to adapt quickly to evolving trial conditions.
Challenges in Traditional Clinical Project Management
Despite the strategic importance of clinical development, many organizations operate with fragmented, manual tracking systems. Key challenges often include:
- Dispersed data across sponsors, CROs, and sites, limiting real-time visibility into study progress.
- Manual updating of milestones, site activations, monitoring visits, and enrollment progress, leading to delays and inconsistencies.
- Weak cross-functional collaboration across clinical operations, regulatory, data management, safety, and vendor teams.
- Limited ability to predict timeline risks or operational bottlenecks in advance.
- Complexity in managing multi-region trials with varying regulatory requirements and vendor ecosystems.
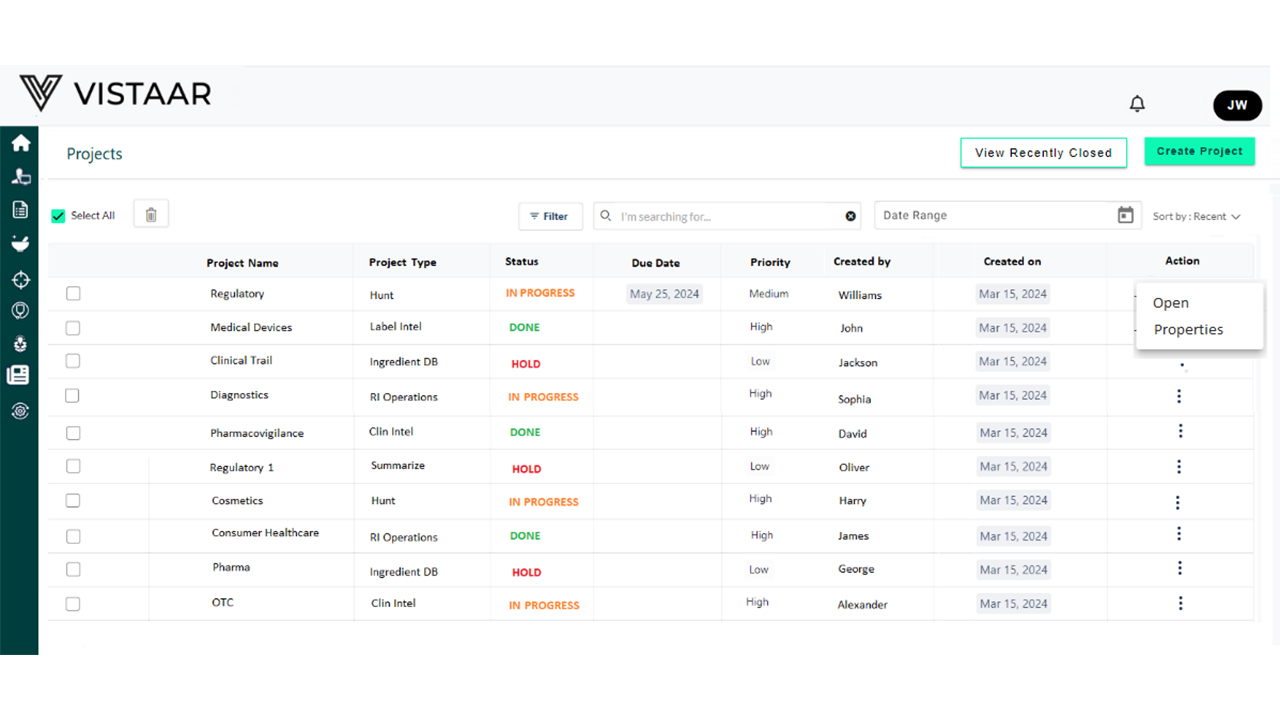
DDi’s Clinical Project Management & Tracking Platform
DDi’s platform replaces manual, disconnected processes with intelligent, automated, and unified trial oversight. It is purpose-built for pharma and biotech clinical workflows and integrates tracking, collaboration, analytics, and compliance readiness into a single operating environment.
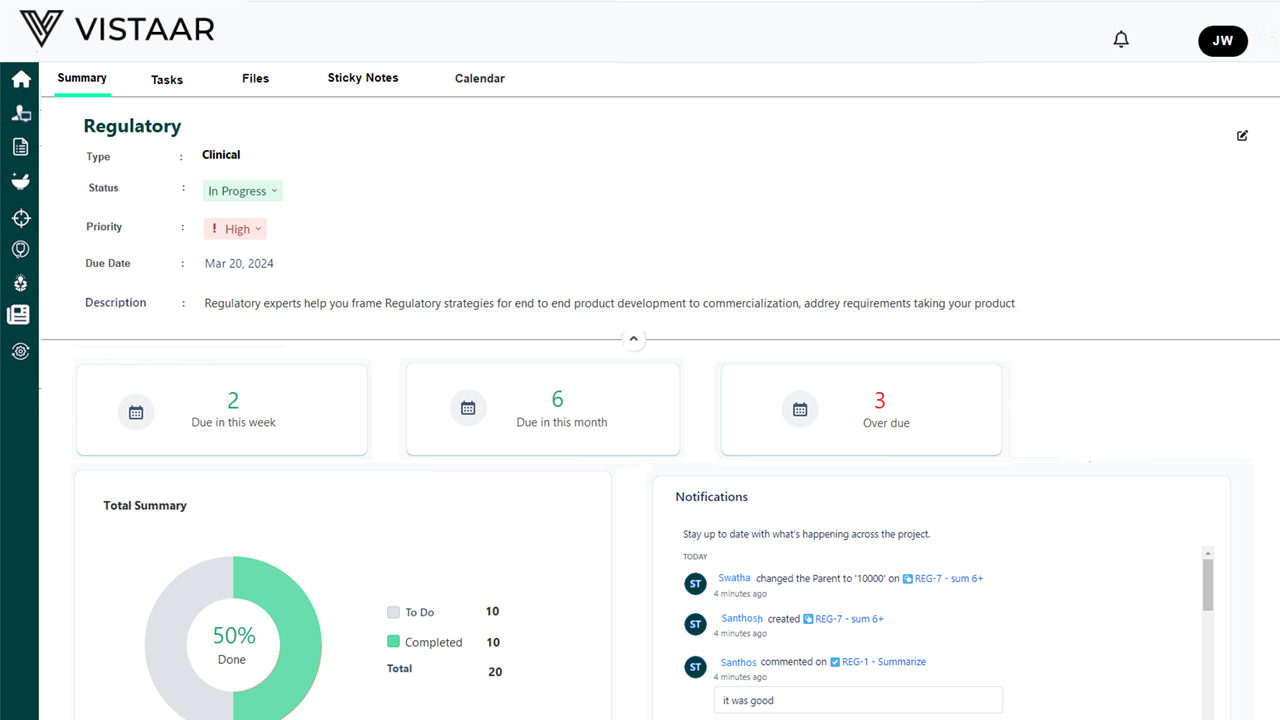
Real-Time Milestone & Site Tracking
The platform provides a consolidated dashboard for all key milestones – including site initiation, enrollment targets, monitoring cycles, database lock, and safety review boards. Dynamic Gantt charts and visual dependency mapping automatically reflect changes, enabling teams to always work from the latest information.
Automated Task & Resource Intelligence
Machine learning models detect priority actions, highlight overdue tasks, and identify resource constraints across sites, teams, or vendors. This helps operations teams plan better and act faster, without relying on manual follow-up chains.
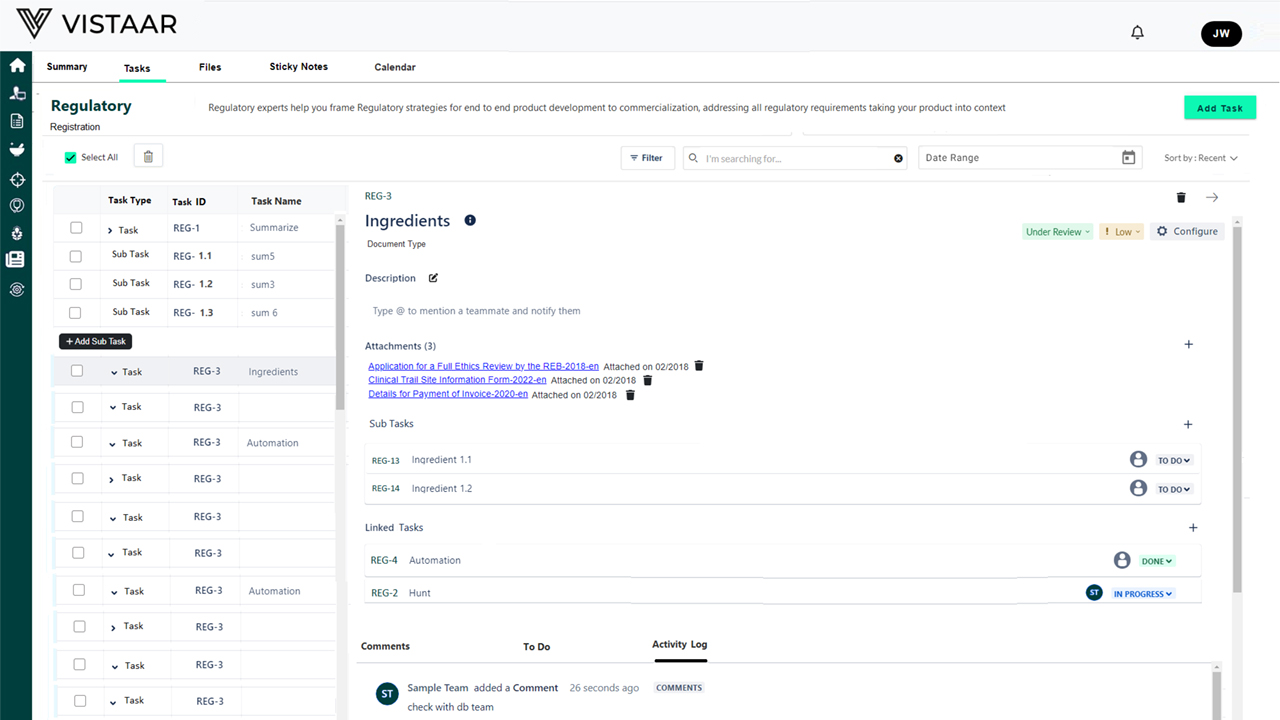
Cross-Functional Collaboration Hub
Role-based access ensures clinical operations, regulatory, data management, safety teams, vendors, and leadership all work from a single source of truth. Task assignments, document versions, audit trails, and communication flows are centralized for transparency and control.
Predictive Risk & Timeline Analytics
AI-driven forecasting anticipates potential delays due to enrollment shortfalls, site inactivity, vendor performance issues, or protocol amendments. Teams receive early alerts to intervene proactively, rather than reactively.
Seamless Integration with Data & Regulatory Workflows
The platform connects project tracking with data capture, safety events, reporting, and submission readiness, reducing hand-offs and ensuring traceability across the clinical lifecycle.
Key Benefits:
- Accelerated Study Start-Up and Execution Real-time site and milestone visibility shortens site activation cycles and improves enrollment pace.
- Enhanced Compliance and Audit Preparedness Every action, document, milestone, and deviation is version-controlled and traceable, ensuring inspection readiness.
- Reduced Manual Workload and Operational Overhead Automation eliminates repetitive status updates, report compilation, and reminder communications.
- Efficient Oversight of Global, Multi-Site Trials Standardized workflows simplify coordination across geographies, vendors, and regulatory landscapes.
- More Informed, Data-Driven Decisions Predictive analytics and risk forecasting enable proactive intervention and continuous optimization.
Built-in AI & Automation Capabilities That Drive Performance:
- Natural Language Processing (NLP) extracts key metadata from clinical documents to streamline tracking.
- Machine Learning (ML) optimizes site selection, vendor performance, resource allocation, and timeline planning.
- Predictive Analytics anticipates enrollment risks and operational delays before they impact milestones.
- Agentic AI Assistants provide intelligent recommendations for escalation, prioritization, and corrective actions.>L/li>
Why Partner with DDi?
DDi combines deep life sciences domain expertise with advanced clinical technology. Our platform is purpose-built, not repurposed, and supports enterprise-scale global trials with:
- Proven implementation success across complex pharma and biotech environments
- Integrated clinical, regulatory, and operational workflows
- High configurability, scalability, and security
Contact us to schedule a demo and explore how we can transform your clinical operations.
Let's talk about how DDi can help you


