Submission & Publishing Management
for Medical Device
Automate, Accelerate, and Stay Compliant
Regulatory operations in the medical device industry demand precision, speed, and alignment with evolving global standards. Our advanced Submission & Publishing Management solution for med devices empowers regulatory teams to streamline end-to-end processes — from document collection to submission lifecycle updates — with high accuracy and automation.
Key Capabilities of Our Publishing Management Software for Medical Devices
Document Collection
Collect documents from cross-functional teams or external systems. Accepts multiple formats like Word or PDF. Our system automatically checks quality and applies formatting fixes to meet country-specific submission requirements.
Document Preparation
Our solution automates formatting, editing, and reviewing — saving teams hours of manual work. Document readiness is handled through built-in automation rules tailored for medical device submissions.
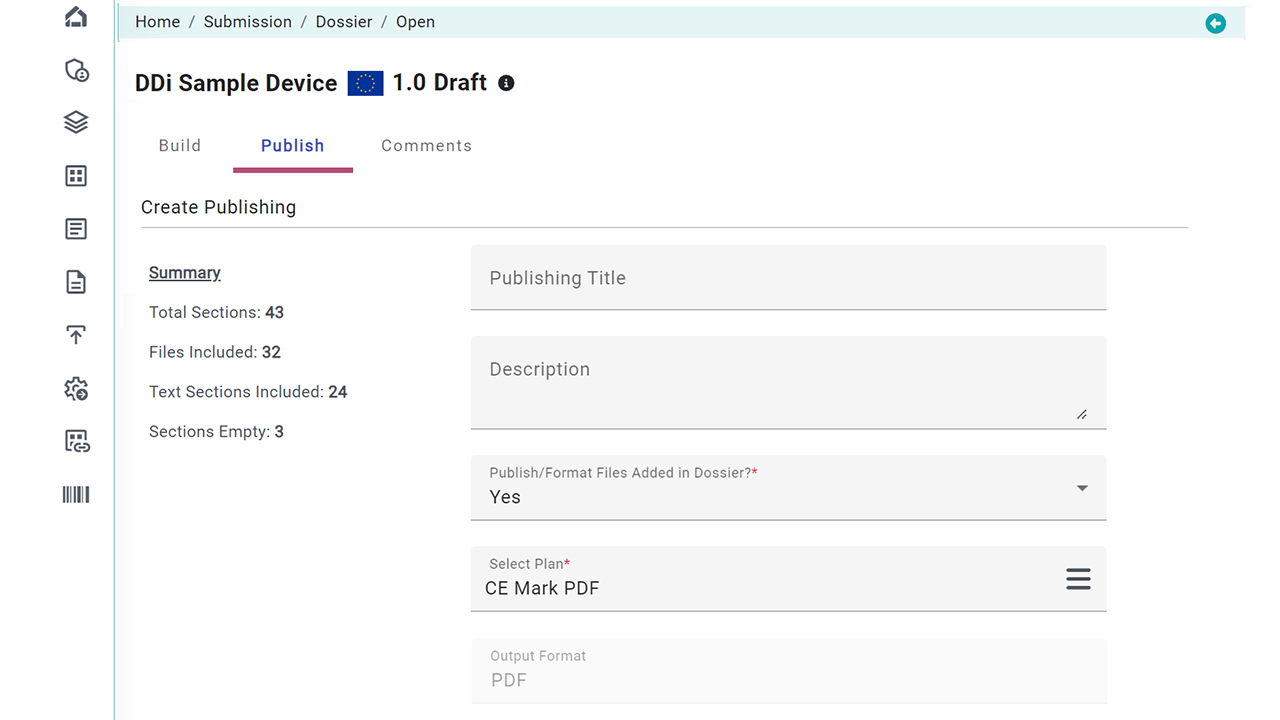
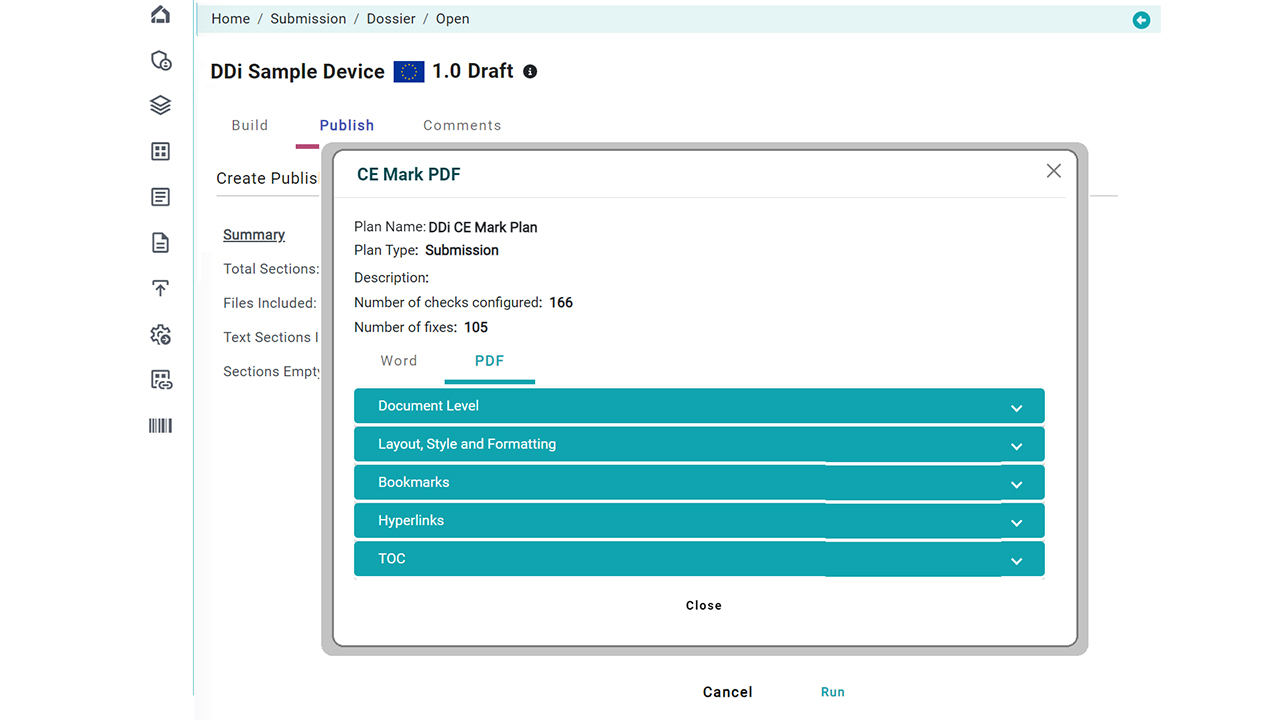
Document Publishing
Once prepared, documents are converted into compliant, submission-ready formats using our built-in publishing engine. Create final packages per region (FDA, EU MDR, etc.) with a click.
Submission Tracking
Track submission status, receive health authority acknowledgments, respond to information requests, and monitor progress — all from one centralized dashboard.
Life-Cycle Updates
Easily manage post-approval updates such as label modifications or manufacturing changes. Our lifecycle module supports both document-level updates and related correspondence.
Regulatory Compliance
Built-in compliance with ICH, FDA, EU MDR, and your SOPs ensures your documents meet global requirements without added complexity.
Collaboration & Workflow Management
With integrated project management and real-time collaboration tools, teams can work together seamlessly across functions including RA, QA, clinical, and regulatory ops.
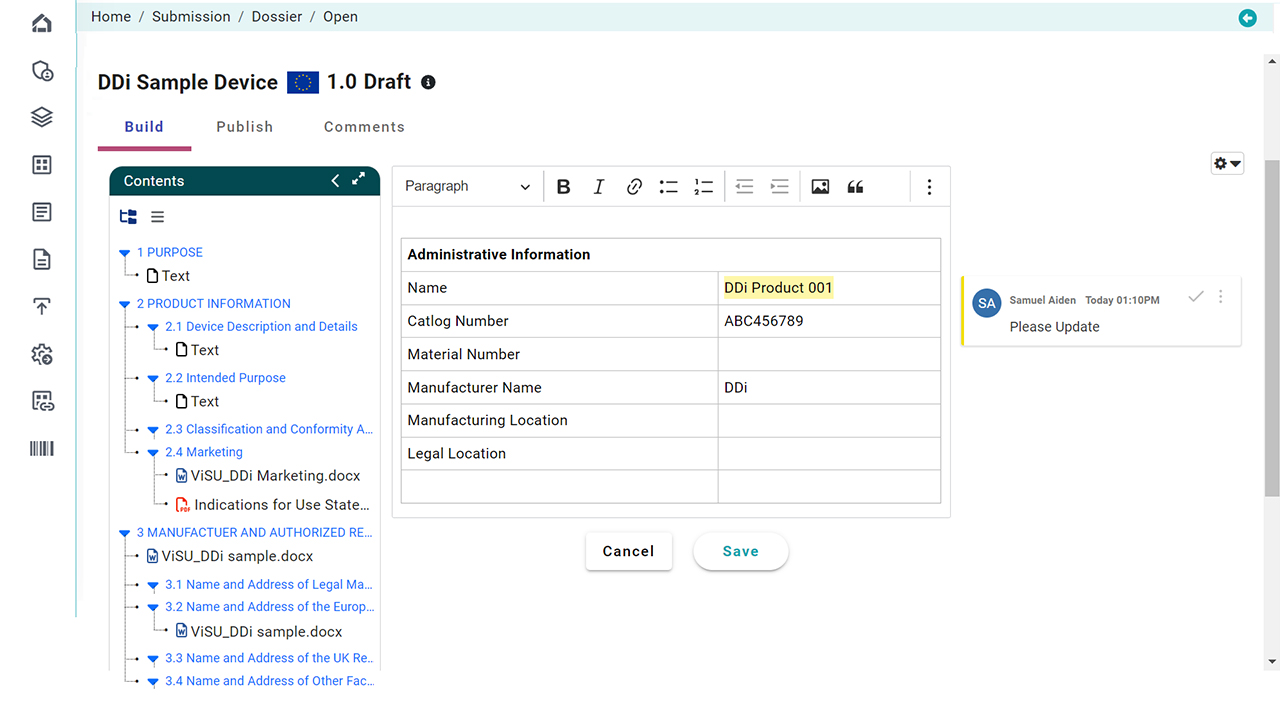
Document-Level Publishing Made Simple
Publishing shouldn’t be painful. Our solution automates every step of document publishing for med devices, regardless of file format. Whether your source is Word or PDF, generate submission-ready documents in seconds using 300+ customizable rules.
- Format documents based on FDA, EU, or custom guidelines
- Apply TOC, hyperlinks, headers/footers, and more automatically
- Centralized rule library gives you full flexibility
- Control publishing flow via simple, intuitive UI
Result? Over 70% cost and time savings — guaranteed.
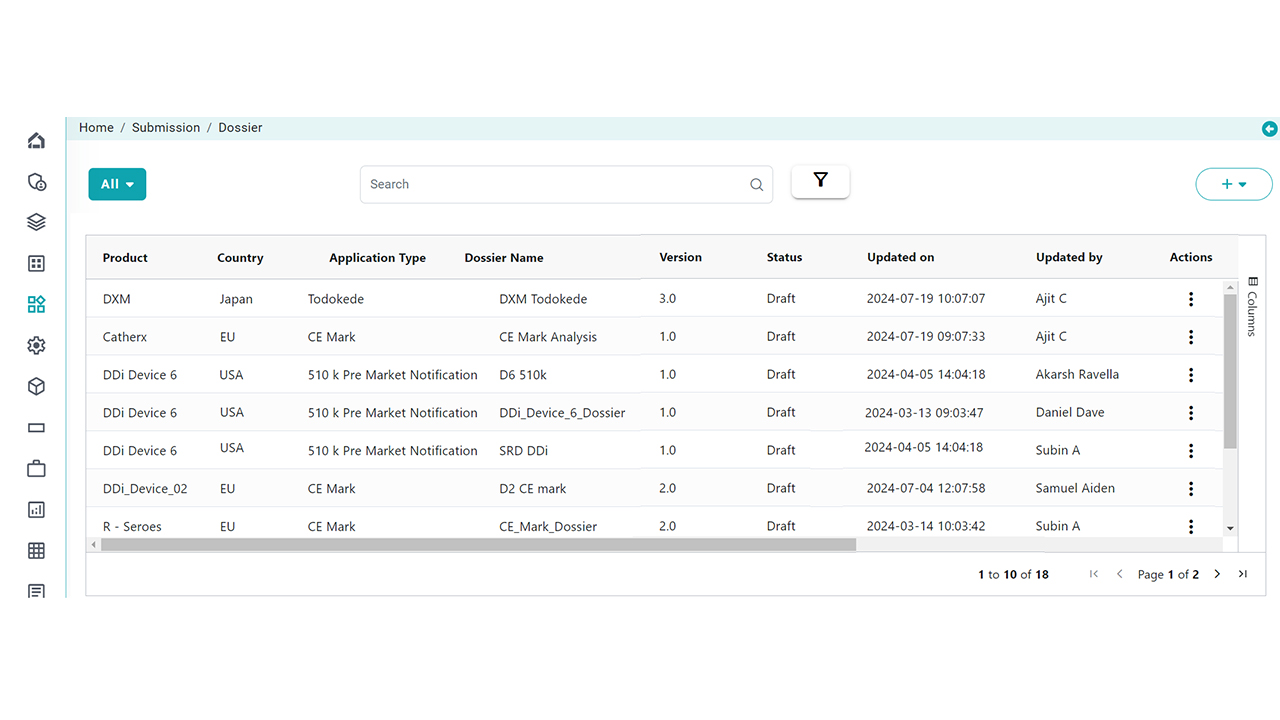
Dossier Build & Publish Capabilities
Whether you’re managing new submissions or life cycle updates, our platform supports end-to-end dossier creation and publishing for global markets.
Highlights:
- Pre-built country-specific publishing templates
- Custom submission plans with flexible template management
- Manage all submission types from a single dashboard
- Cross-country dossier linkage support
- Output formats include XML, ZIP, and PDF
- Full integration with your EDMS, PLM, or ERP
Why Choose Our Submission & Publishing Management Software for Medical Devices?
- Reduce cycle time and manual effort
- Maintain compliance with built-in country-specific rules
- Gain full control of your submission workflows
- Increase transparency and team collaboration
- Cut publishing costs with automation
Ready to simplify Submission & Publishing Management for med devices? Our platform delivers the agility and compliance your team needs to thrive in a dynamic regulatory environment.
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you


