Regulatory Project Management & Tracking
for Medical Devices
Accelerate Compliance and Innovation with AI-Driven Project Management
In today’s fast-paced Medical Device / Diagnostic landscape, staying compliant with evolving global regulatory requirements is more demanding than ever. Medical device companies must juggle complex submissions, documentation cycles, and multi-market launches – often across jurisdictions like FDA (U.S.), MDR (EU), and ISO 13485 (global).
DDi’s AI-enabled regulatory project management platform empowers medical device companies to streamline compliance, reduce errors, and bring products to market faster – all while staying audit-ready.
The Problem: Regulatory Complexity, Manual Burden
Traditional project management approaches rely heavily on manual tracking, spreadsheets, emails, and disconnected systems – introducing major limitations:
- Human errors in document versioning or submission timelines
- Delays in approvals due to inefficient workflows
- Lack of real-time visibility for project stakeholders
- Difficulty adapting to changing global regulations
These gaps can slow down product launches and increase compliance risks.
How DDi Modernizes Regulatory Project Management with AI
Our intelligent regulatory management platform is designed specifically for medical device companies, combining automation, predictive analytics, and compliance insight to optimize every stage of your regulatory journey.
Intelligent Document Automation
AI-powered tools scan, extract, tag, and validate regulatory documents (e.g., STED, CERs, labeling, technical files) with unmatched speed and consistency.
- Auto-tagging, classification, and version control
- NLP-based formatting and validation
- Alignment with FDA, MDR, and ISO 13485 templates
Predictive Risk Forecasting
Our system learns from past submissions to highlight potential delays, bottlenecks, or missing elements – before they become roadblocks.
- Approval timeline prediction
- Workflow risk alerts
- Inconsistency detection in submission packets
Workflow Automation & Deadline Tracking
Streamline regulatory workflows with Robotic Process Automation (RPA). From form-filling to stakeholder alerts, DDi ensures every deadline is tracked and met.
- Smart milestone calendars
- Auto-reminders and escalation triggers
- Submission status dashboards
Real-Time Global Regulatory Intelligence
Monitor and adapt to ongoing changes in regulatory policies worldwide.
- Real-time updates from FDA, EU MDR, PMDA
- Dynamic compliance checklists
- Localized submission strategies for multi-region launches
Built-In Audit Readiness
Maintain a complete audit trail, document history, and traceability logs to ensure you’re always prepared for inspections.
- Export-ready reports
- Role-based access controls
- Change logs with version traceability
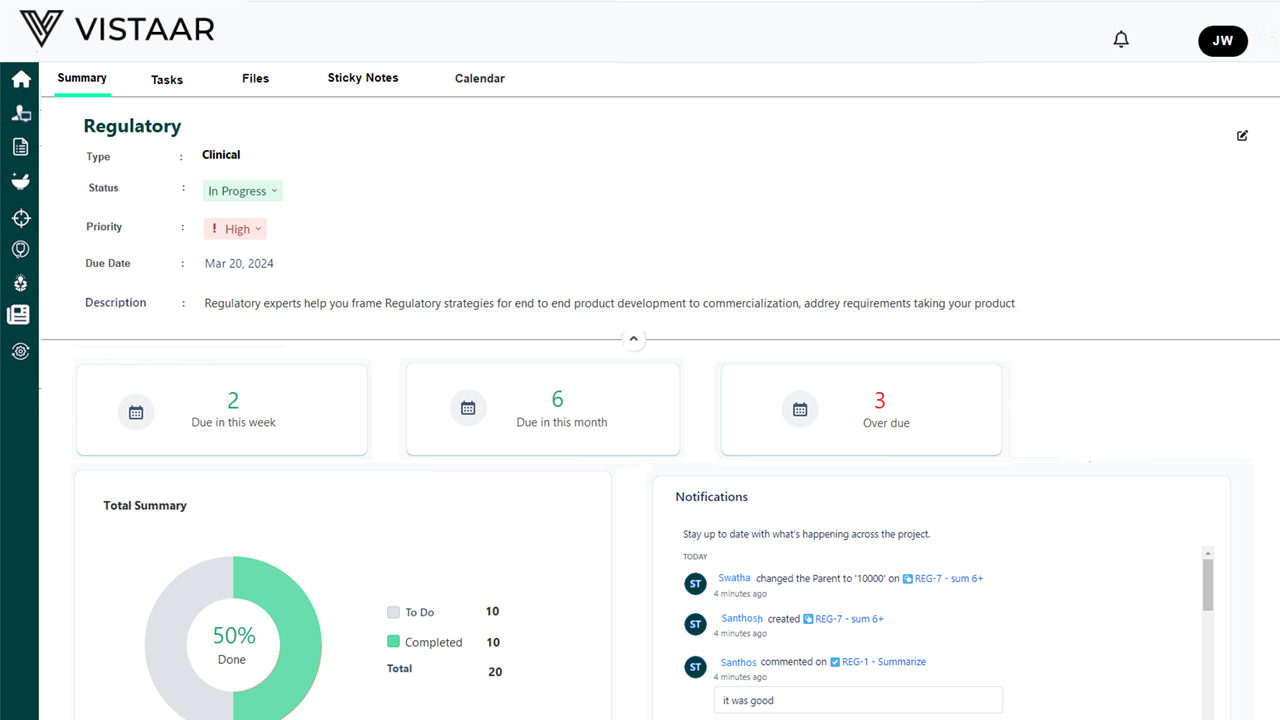
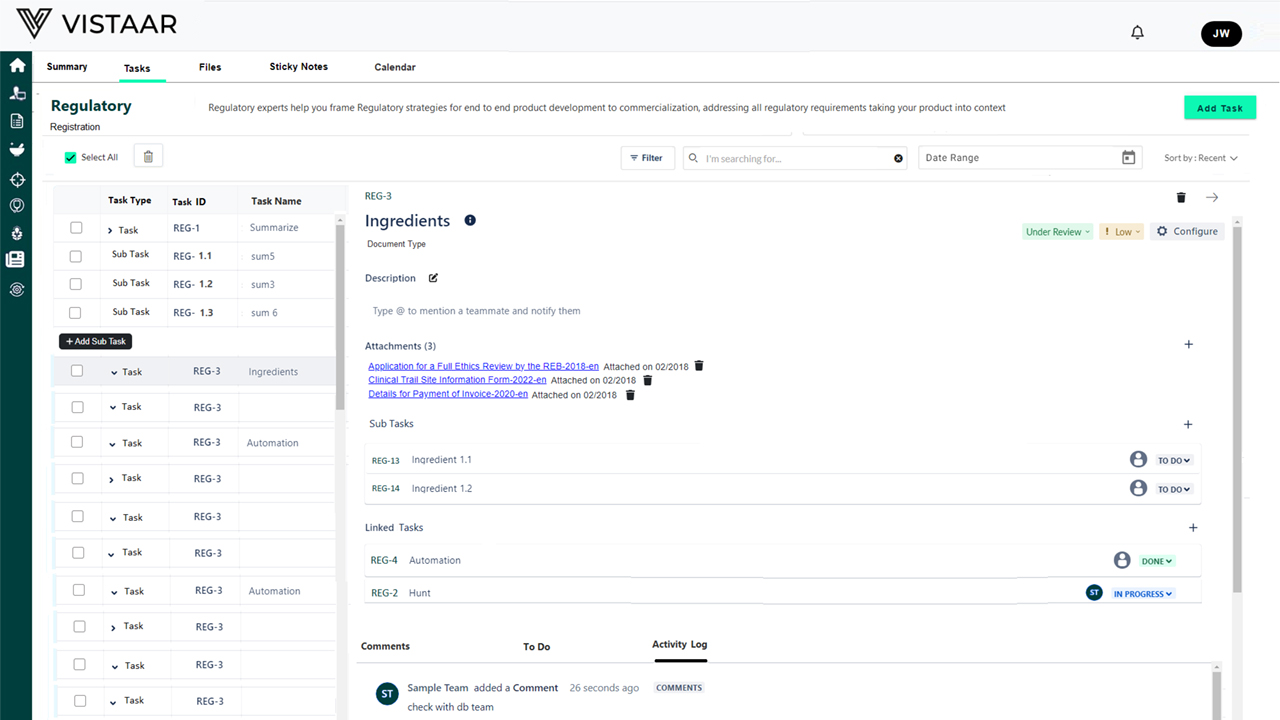
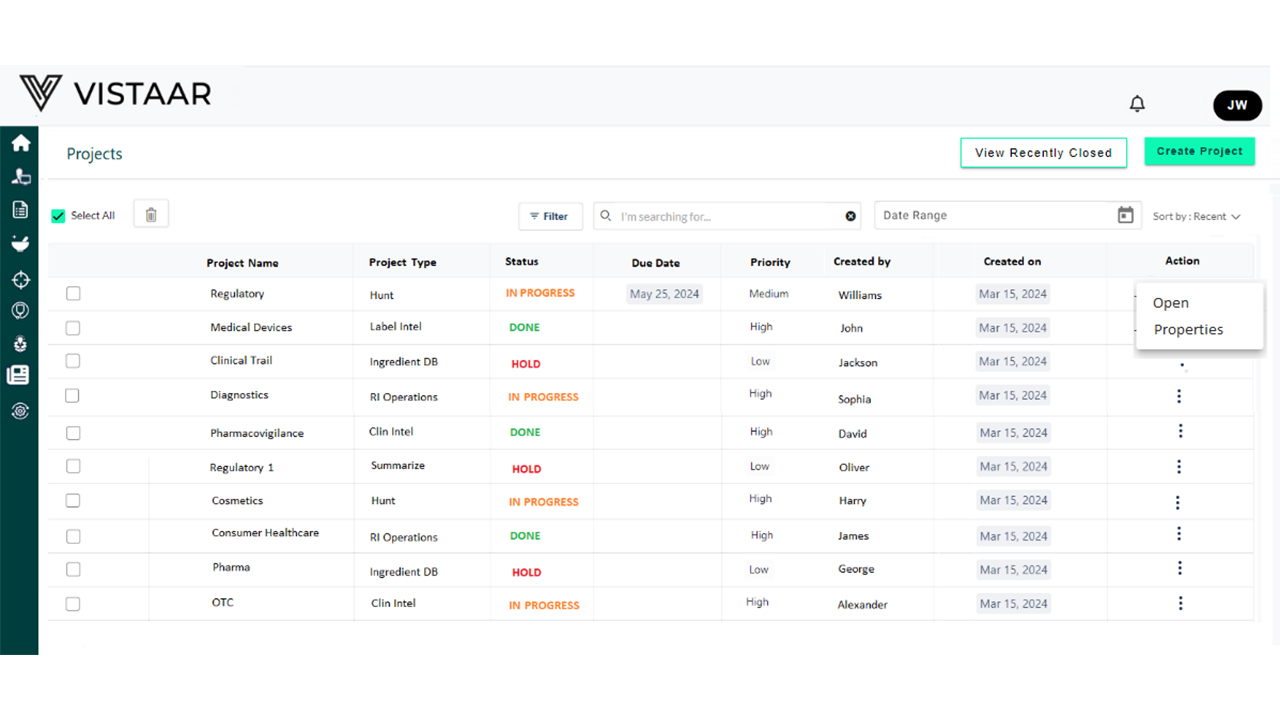
Benefits of Using DDi for Medical Device RPM
- Faster Approvals: Predictive workflows and automation reduce delays
- Improved Accuracy: Minimize manual errors and rework
- Scalable Compliance: Manage global projects from a unified platform
- Always Audit-Ready: Ensure clean documentation trails and version control
- Data-Driven Decisions: Gain actionable insights into risks and performance
Future-Proof with AI-Driven Compliance
Tomorrow’s Medical Device / Diagnostic innovators will need more than spreadsheets to stay competitive. With AI-enabled regulatory management, expect to see:
- Adaptive AI that learns from each submission
- Voice-activated compliance tools
- Instant alerts for regulation changes
- AI-led response simulations for regulatory authority expectations
With DDi, the future is already here.
Ready to Revolutionize Your Regulatory Workflows?
Whether launching a Class I device or navigating EU MDR, DDi’s platform ensures smarter, faster, and more compliant regulatory execution.
Book a Demo or connect with our Medical Device Automation experts today
Let's talk about how DDi can help you


