Medical Writing QC Automation Solutions
for Pharma Biotech
Accelerate Accuracy and Compliance
With the increase in regulatory expectations and submission timelines getting shorter, quality control in medical writing has become more complex and resource-intensive. Traditional manual QC processes are no longer sufficient to handle growing document volumes, evolving global regulations, and increasing scrutiny from health authorities.
At DDi, we understand that errors in medical documents can lead to regulatory questions, submission delays, rework, and reputational risk. Even minor inconsistencies in tables, figures, or references can trigger agency queries. Our Medical Writing QC Automation solution delivers the speed, accuracy, and regulatory confidence you need to stay ahead.
The Challenge: Limitations of Manual QC
While human review remains essential, manual QC alone presents several challenges.
- Time-Consuming: Reviewing lengthy clinical documents line by line slows down submission timelines and increases pressure on medical writers.
- Human Error: Fatigue, repetitive checks, and tight deadlines can lead to missed inconsistencies or overlooked formatting issues.
- Lack of Standardization: Different reviewers may apply QC rules inconsistently, leading to variable quality across documents and projects.
- Scalability Issues: As organizations expand globally, maintaining consistent QC manually becomes increasingly complex.
Our Solution: Intelligent Automation and AI
Automation introduces rule-based checks and AI capabilities that systematically validate documents against predefined standards. Our platform allows medical writers and QC reviewers to focus on higher-value scientific and regulatory assessments rather than mechanical tasks by automating repetitive checks.
Key capabilities include:
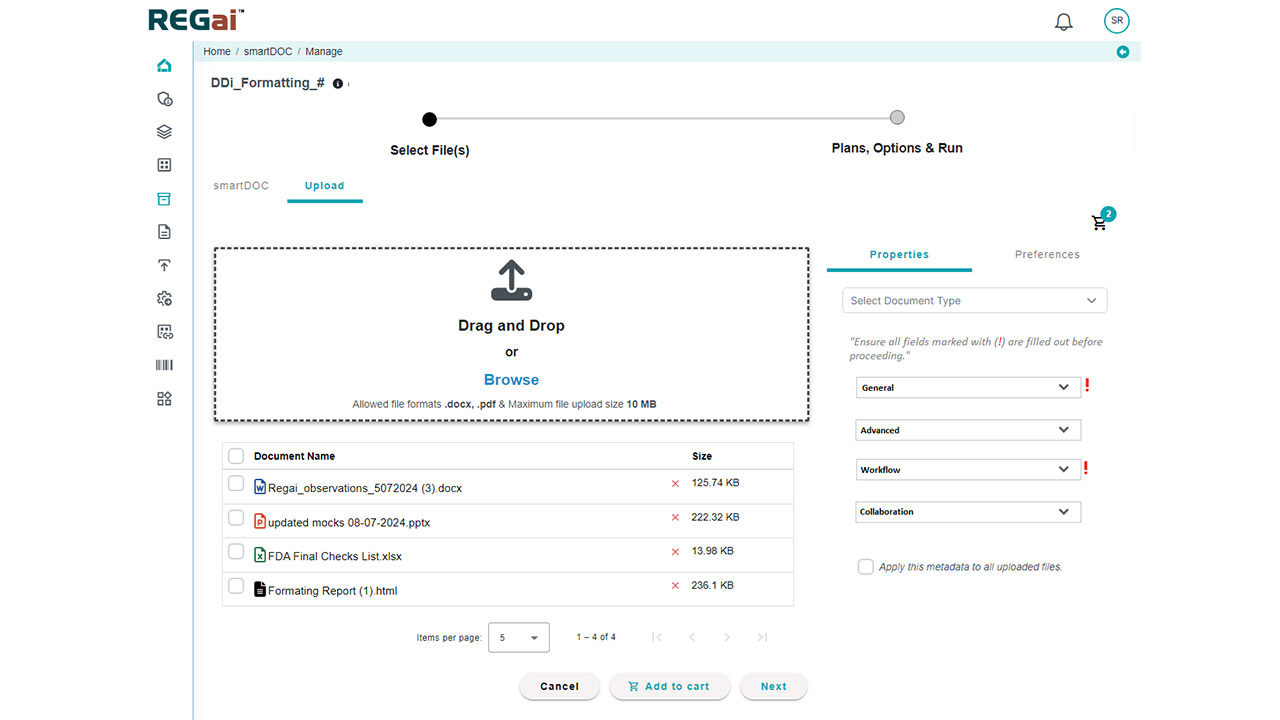
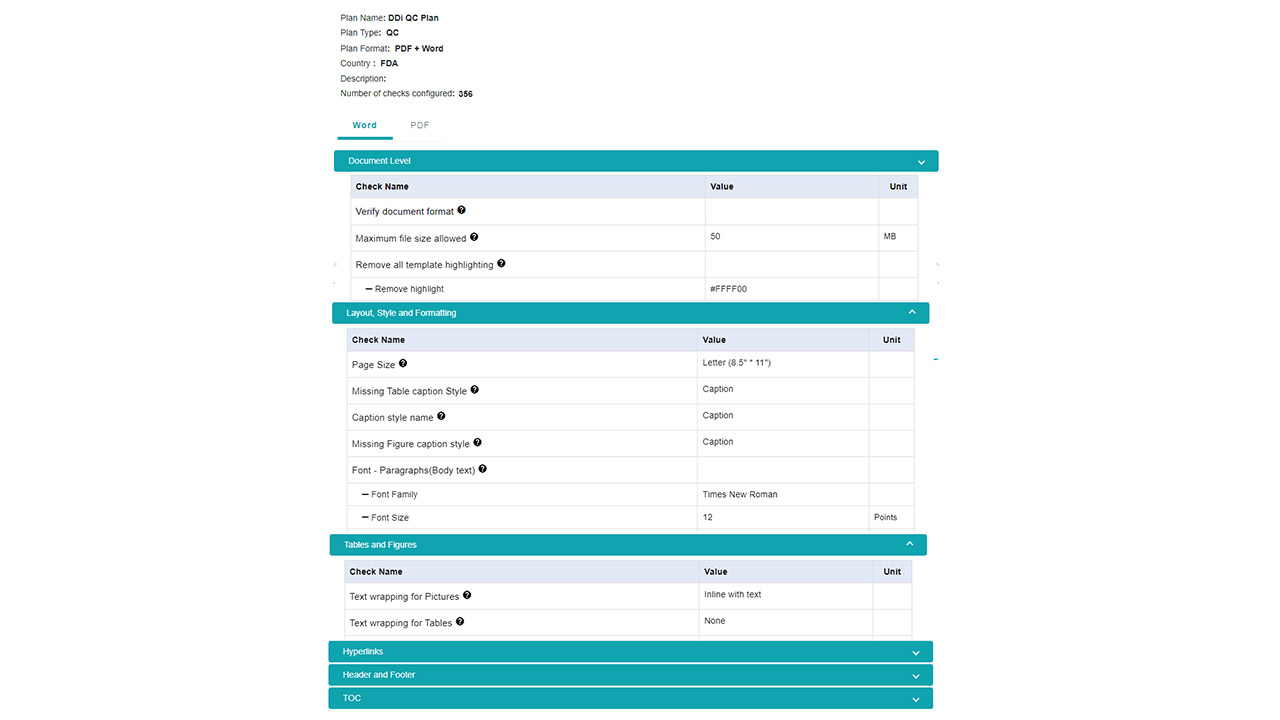
- Template Compliance: Ensure documents follow organizational standards and regulatory expectations every time.
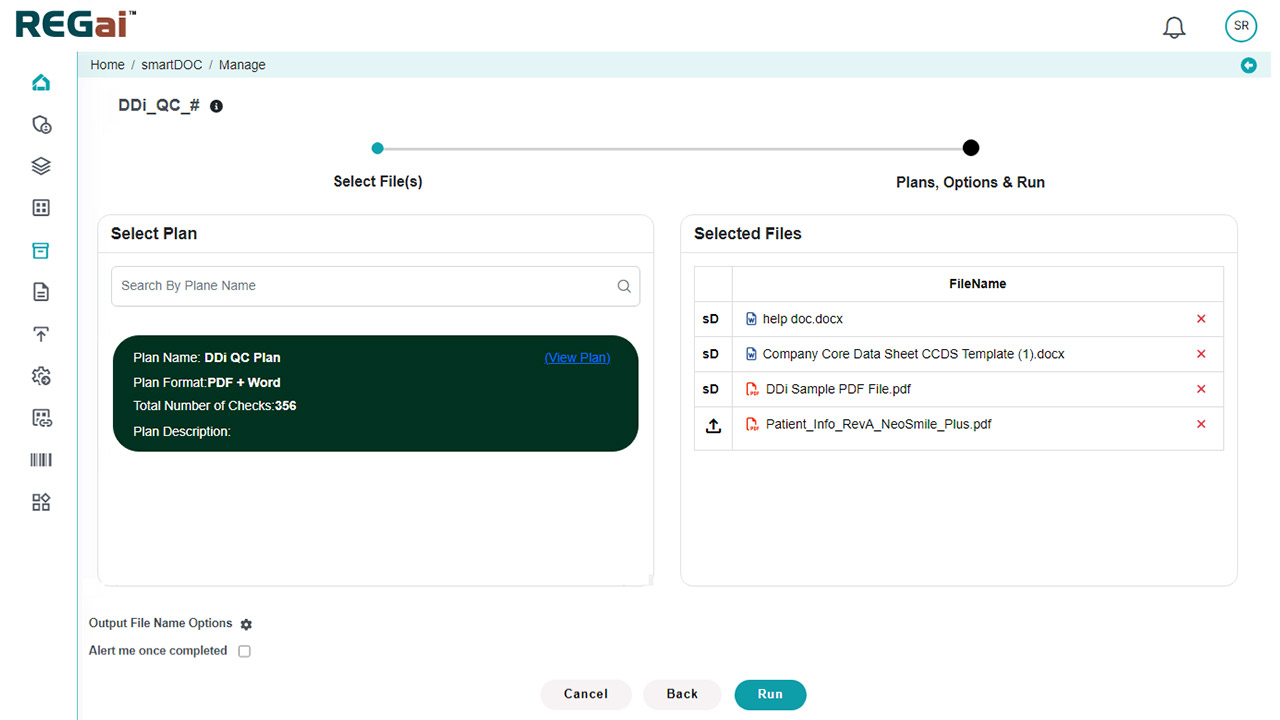
- Rapid Scanning: Instantly scan documents for hundreds of parameters that would take hours to review manually.
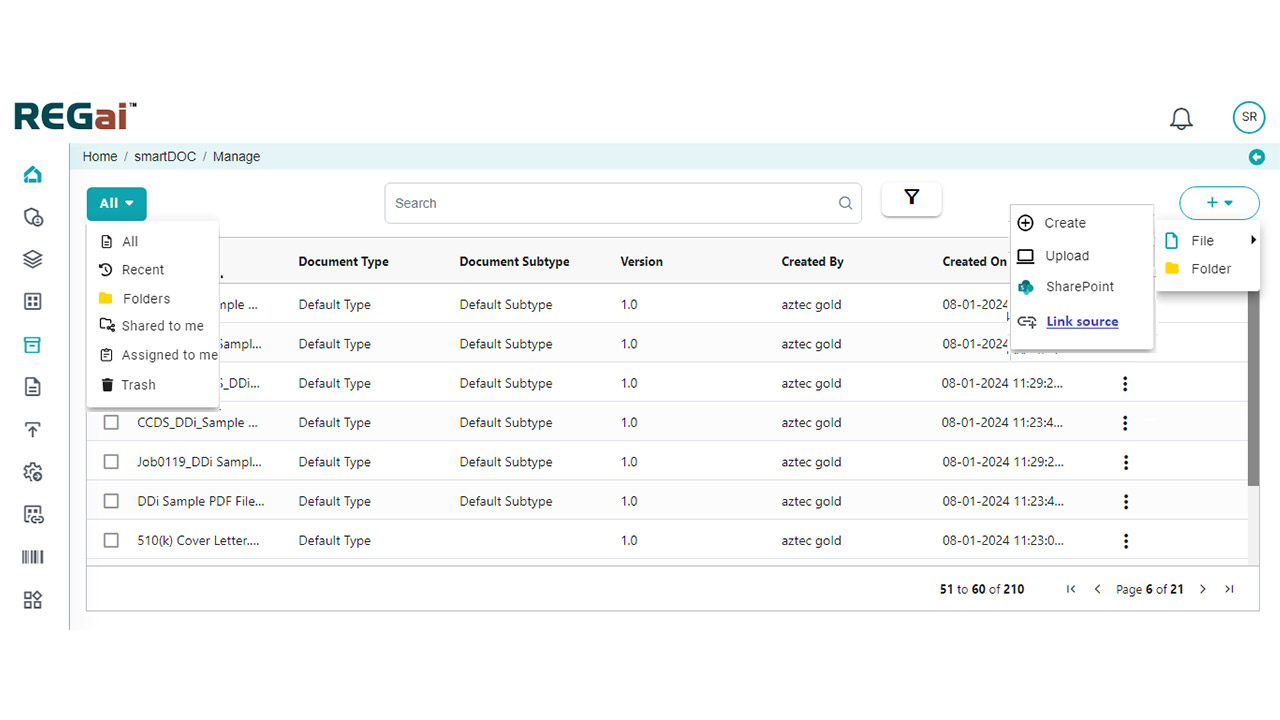
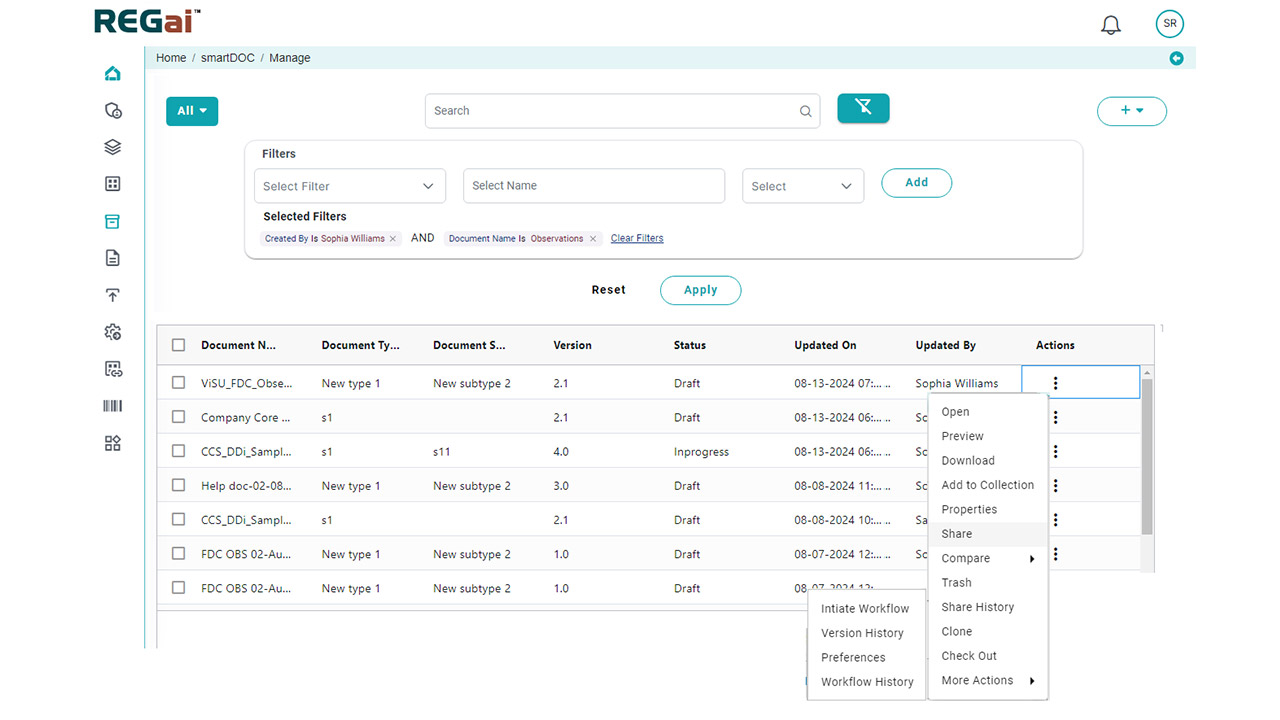
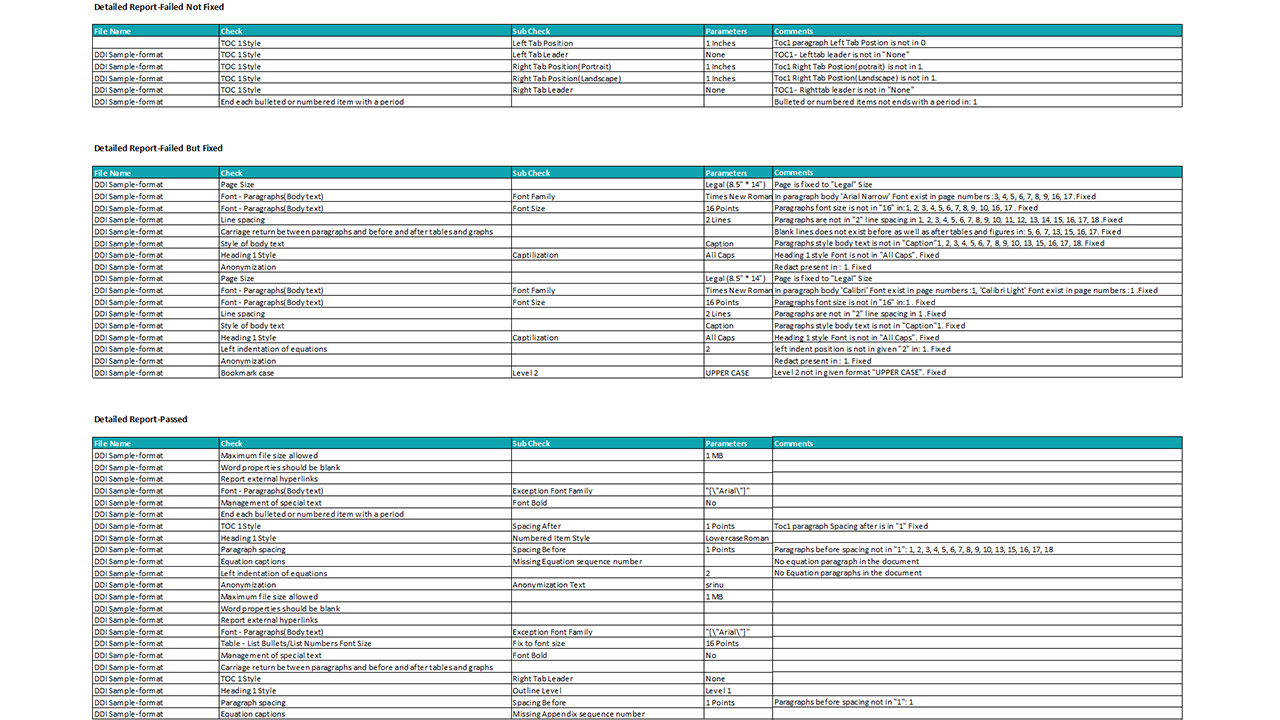
- Comprehensive Validation: Automate checks for data consistency, formatting, style guide adherence, reference validation, and table/figure verification.
Content QC
Automation & AI helps identify inconsistencies in terminology usage, detects logical gaps, flags conflicting data across sections, and highlights deviations from regulatory writing best practices. Unlike static rule-based systems, our AI models continuously improve by learning from historical documents, agency feedback, and organizational preferences.
AI also supports cross-document QC by comparing multiple documents within a submission to ensure alignment across protocols, clinical study reports, investigator brochures, and summaries.
Benefits of Automating Your QC Process
Transform your medical writing process with technology-enabled QC:
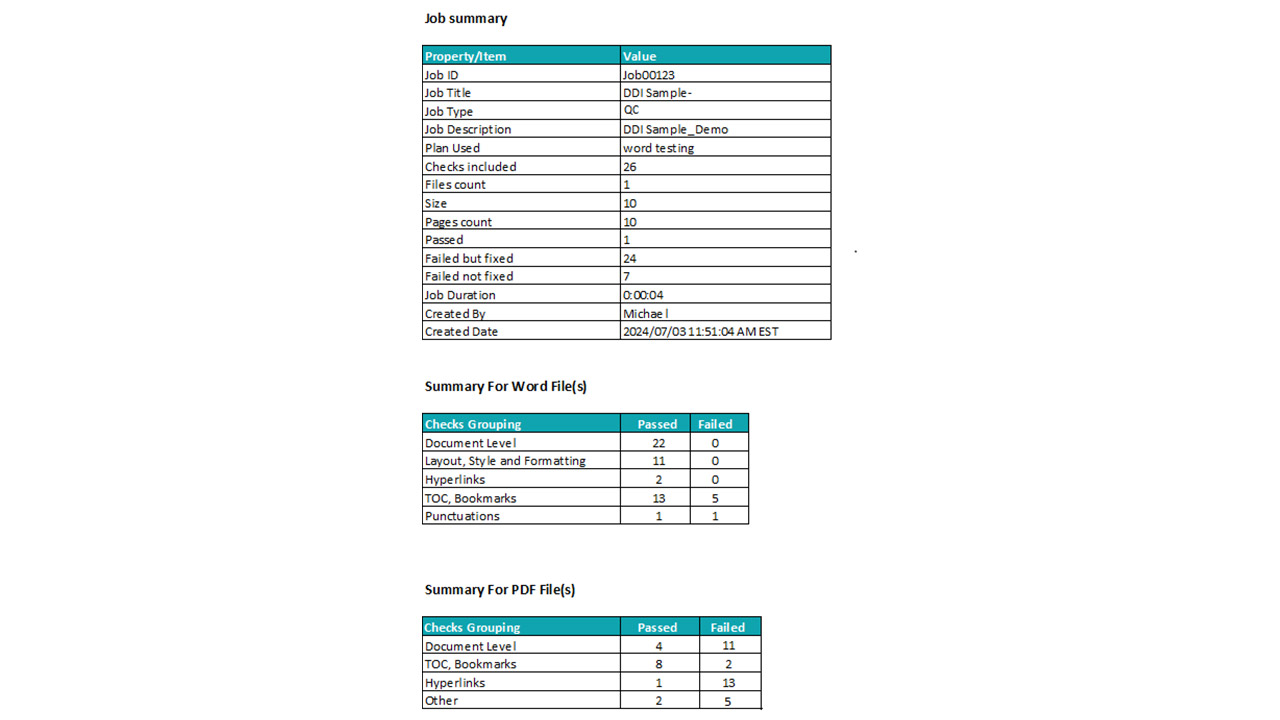
- Improved Accuracy: Automated and AI-driven QC reduces the risk of errors that can lead to regulatory questions or submission rejection.
- Faster Turnaround: Automated checks run in minutes, accelerating document finalization and enabling teams to meet aggressive submission deadlines.
- Global Consistency: Ensure uniform application of QC standards across teams, geographies, and projects.
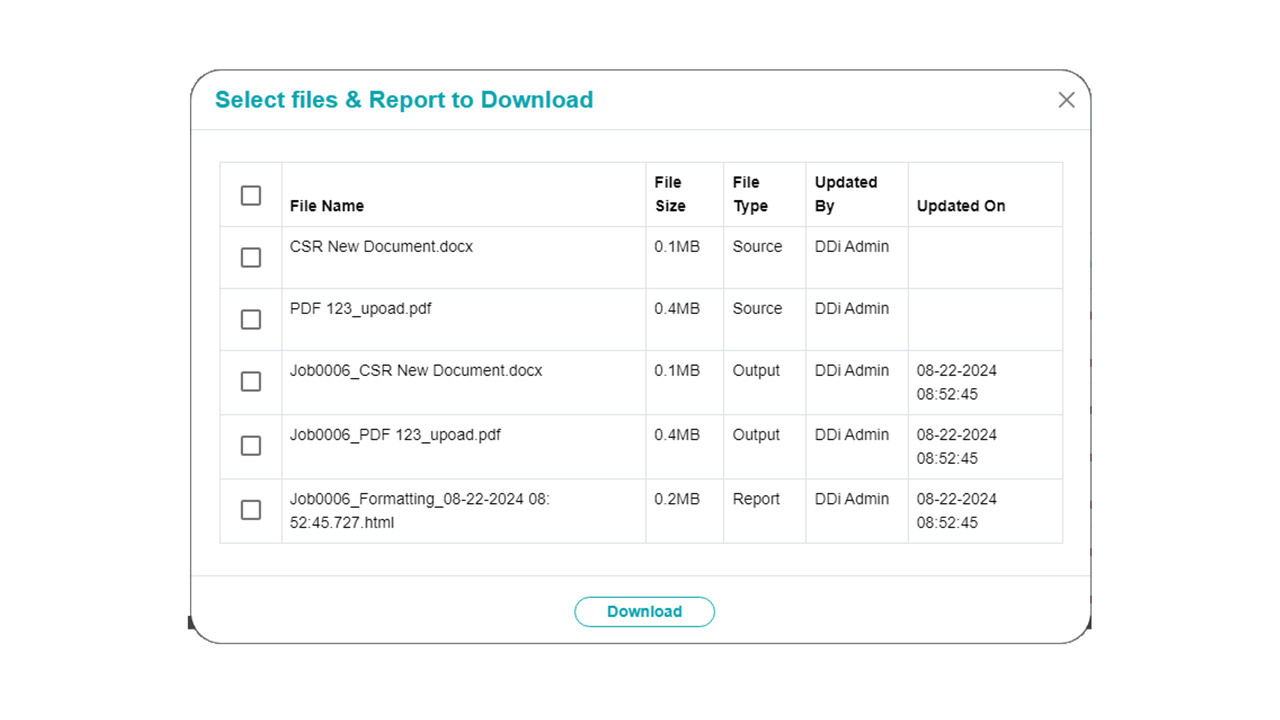
- Audit Readiness: QC activities are traceable, repeatable, and documented, supporting inspection preparedness and quality audits.
Ready to Transform Your QC?
Stop letting manual reviews slow down your submissions and increase regulatory risk. By combining rule-based automation with advanced AI, your organization can achieve faster timelines, greater consistency, and improved regulatory outcomes.
Partner with DDi to:
- Automate repetitive checks so your experts can focus on high-value scientific assessments.
- Ensure total compliance with ICH guidelines and agency-specific requirements.
- Validate both formatting and content to eliminate logical gaps and data conflicts.
Empower your team with the speed, accuracy, and regulatory confidence of AI-driven QC
Contact Us Today for a Demo
Let's talk about how DDi can help you


